Document Type : Original Article
Authors
1 Research Assistant in the Islamic Azad University Pharmaceutical Sciences Branch, Tehran, Iran
2 Lecturer in Department of Biotechnology, Malek Ashtar University of Technology (MUT), Tehran, Iran
3 Department of Cellular and Molecular Biology, Faculty of Sciences, Higher Education Institute of Rab-Rashid, Tabriz, Iran
4 Department of Educational Science and Psychology, Islamic Azad University Marvdasht Branch, Marvdasht, Iran
Abstract
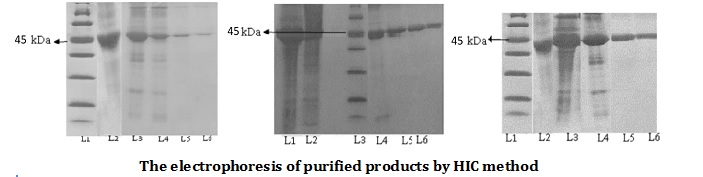
Protein purification has always been one of the most critical and challenging stages of drug-protein production. Streptokinase as the most common cost-effective fibrinolytic drug is no exception. In this research study, the mutated streptokinase producing clone (SK263cyc) to which the histidine tag was grown in TY2x medium, and SDS-PAGE assessed protein expression after induction of protein expression. Three different methods did protein purification. In the first one, metal, ion affinity chromatography (IMAC) technique was used. In the second solution, first, by filtration with ammonium sulfate, the purification was carried out, and then by affinity purification, chromatography continued. In the third solution, hydrophobic chromatography was utilized to purify the streptokinase protein. The purity of the ophthalmic purity was 93.2%, and the purity of hydrophobic purity was found to be 90.4%, whereas the combination of pre-treatment with ammonium sulfate and the purity of the ophthalmic method did not achieve more than 88%. The results of this study revealed that, the IMAC method is more suitable as a final method at the process of streptokinase purification than the other two approaches.
Graphical Abstract
Keywords
- Streptokinase
- Recombinant protein
- Affinity chromatography
- Hydrophobic gel purification
- Ammonium sulphate
Main Subjects