Document Type : Original Article
Authors
University of Baghdad, College of Science, Department of Chemistry, Baghdad, Iraq
Abstract
This study describes a rapid and sensitive method for determination of Losartan potassium (LOS-K) in pure and medication forms. The method is depended upon the reaction of LOS-K with Silicotungestic acid in salt medium for the formation of a white precipitate. The new precipitate was monitored and measured using homemade Ayah 6SXI-T-2D Solar cell analyzer in combination with a continuous flow injection technique. Reagent concentration, acid medium, flow rate, sample volume, purge time, and delay coil reaction are all chemical and physical characteristics that have been investigated and optimized. The linear dynamic range of LOS-K was 0.05-2 mMol/L with linearity percentage (r2%) 99.56 %. Limit of detection (LOD) was 3.62 µg/sample and LOQ=18.28 µg/sample. A comparison between developed method and classical methods (UV-Spectrophotometry at λmax= 232 nm) was made. The proposed method was successfully applied for determination of LOS-K in the pharmaceutical samples and can be used as an alternative method.
Graphical Abstract
Keywords
Main Subjects
Introduction
Losartan potassium (LOS-K) is a synthetic angiotensin II receptor antagonist that belongs to the antihypertensive class [1]. Losartan potassium is described chemically as 2-n butyl-4-chloro-5-hydroxymrthyl-1((2-(1H tetrazol-5-yl) (biphenyl-4-4-yl) methyl) imidazole (Figure 1).
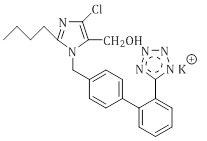
Figure 1: Chemical structure of Losartan potassium
The anti-hypertensives drugs such as losartan potassium are used for combat cardiovascular morbidity [2]. Losartan potassium (LOS-K) is the first new class of hypertension medications to be approved for clinical usage. In general, the metabolite contributes significantly to the antihypertensive impact of the drug, which lasts for 24 hours after administration. [3]. Angiotensin II receptor blockers, such as LOS-K, come in two dose strengths (50 mg and 100 mg tablet), two tablet formulations (film-coated and core), and can be used alone or in combination with hydrochlorothiazide. [3]. LOS-K was the first nonpeptide to be licensed for the treatment of hypertension, either alone or in combination with other antihypertensive drugs, in 1995 [4]. Losartan tablets are used to preserve the kidneys in hypertensive type 2 diabetic individuals with impaired renal function and proteinuria of less than 0.5 grams per day (urine containing an excessive amount of protein). LOS-K can be administered in combination with diuretics [5]. Losartan potassium (LOS-K) is a white to off-white powder with a molecular weight of 462.01 g.mol-1 and a pKa of 4.9, making it extremely soluble in water, isopropyl alcohol, and acetonitrile, and has a melting point of 183.5- 184.5°C; it is available in the pharmaceutical market in form of tablets. LOS-K has the chemical formula of C22H23ClN6O [6]. There are many methods to study and determine LOS-K in pure form and pharmaceutical preparation, which include: Spectrophotometric [7-8], spectrophotometric and HPLC [9], high performance liquid chromatography (HPLC) [10-11], conductimetric [12], spectrofluorimetric [13] and electrochemical and photometric method [14]. The developed method in this research was dependent on the measurement of attenuation turbidimetric method using precipitating agent Silicotungestic acid to the determination of LOS-K in pharmaceutical drug.
Material and methods
Reagent and Chemical
All the chemical compounds employed in this study were of analytical grade and dilutions were done using distilled water. A stock solution 10 mMol/L of Losartan potassium molecular weight 462.01 g/Mol was prepared by dissolving 1.155 g in 250 mL, and 10 mmol/L of Silicotungestic acid molar mass 2878.2 g/Mol was prepared by dissolved 7.196 g in 250 mL of distilled water.
Apparatus
The instrument used in this work was home-made Ayah 6SXl- T-2D Solar cell CFI analyzer which has six snow-white light emitting diode LEDs for irradiation of the flow cell over a 2 mm route length, and two solar cells for signal detection over a 60 mm path length [15]. Peristaltic pump is based on four-channel and variable speed (Ismatec, Switzerland) and six-port and two-direction injection valve with a different sample volume. The output response signals were plotted by using x-t potentiometric recorder (Siemens Kompenso C-1032, Germany) for the developed method while the scattering light for the formed precipitate was measured by two classical methods (UV-Vis Spectrophotometer (Shimadzu model UV-1800, Japan) and Turbidometry via Turbidity-meter, HANNA-Hungary).
Methodology
The design of the two-line manifold system (Figure 2) was used to Ayah 6SXl- T-2D Solar cell analyzer for Losartan potassium (LOS-K) determination using Silicotungestic acid preliminary experimental concentration (0.2 mMol/L) as precipitate reagent to form white precipitate as an ion pair complex. The distilled water (D.W) was used as carrier stream at 1.6 ml/min flow rate, leading to injection valve to carry LOS-K (2 mMol/L) from the sample loop 157 μL and combine with the second line (1.4 ml/min) at Y-junction to form precipitate. The measurements of responses were carried out by Ayah 6SXl- ST-2D Solar, which was recorded via used x-t potentiometric recorder (Siemens Kompenso C-1032, Germany). The proposed mechanism of the reaction between Losartan potassium and Silicotungestic acid is illustrated in Scheme 1 below [16].
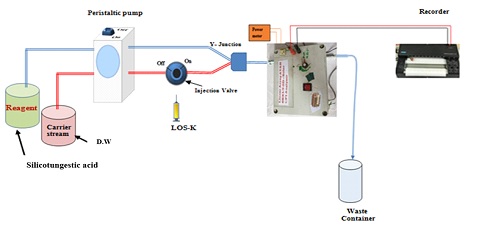
Figure 2: Two-line manifold system design for determination of LOS-K(2 mMol/L) using Ayah 6SXl- T-2D Solar cell analyzer
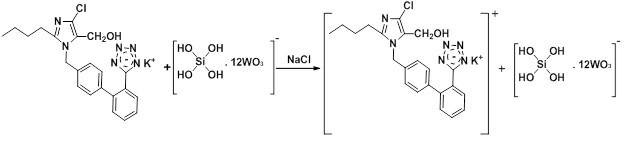
Scheme 1: Proposed mechanism of the reaction between Losartan potassium and Silicotungestic acid
Result and Dissection
Optimization of Reaction Pattern Parameters
Chemical as well as physical parameters affecting the sensitivity of a clear profile of response were investigated. The chemical variables are the concentration of Silicotungestic acid as reagents in presence of several acid kinds and salts. In addition, the effect of type medium on the signal response and sensitivity was studied. The physical variables involved: Flow rate, sample volume, purge time and coil length.
Chemical Variables
Precipitating reagent (Silicotungestic acid) concentration
The study was carried out using a variable concentration of precipitating reagent Silicotungestic acid ranging 0.1-2 mMol/L. The Silicotungestic acid reagent was prepared and transferred at 1.4 ml/min flow rate, with 1.6 ml / min flow rate of carrier stream (D.W), 157 μL sample volume using open valve mode and 2 mMol/L concentration of LOS-K. The applied voltage to the LEDs was 2-volt DC. As shown in Figure 3, it was found that the responses profile increased when the precipitate agent increased up to 0.5 mMol/L due to formation of precipitate particulate that might have increased the reflection of incident light, which refers to increase on incident light intensity. Three times, each measurement was taken. There was a decrease in light intensity when the concentration was greater than 0.5 mMol/L, which might be due to the formation of large agglomerate particles, or a precipitate with large particles, leading to the formation of interstitial spaces that pass a larger amount of focused light and thus reduces the response. Therefore, 0.5 mMol/L were selected as the optimum concentration.
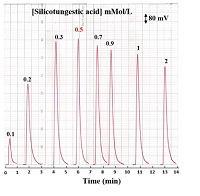
Figure 3: Response – time profile of LOS-K with variable concentration of Silicotungestic acid using 2 mMol/L for LOS-K with 157 μL sample volume
Effect of reaction medium
Series of different medium solutions at 10 mMol/L of H2SO4, HCl CH3COOH and 15 mMol/L KBr, KNO3, NH4Cl and NaCl as carrier stream were applied, in addition to distilled water for analysis LOS-K by reaction with Silicotungestic acid. Another condition was flow rate 1.6ml/min for carrier stream and 1.4ml/min for reagents; 2 mMol/L of LOS-K was used as the injected concentration and 157μL sample volume. An increase of peak height and sharp response in presence of NaCl as carrier stream was noticed. Most probably, another medium (H2SO4, HCl, H3PO4, KBr, KNO3, NH4Cl and NaCl) causing dispersion of precipitate particulate into a smaller one, was in turn unable to reflect all light [17]. Therefore, NaCl was the optimum choice to use for LOS-K- Silicotungestic acid system. All the obtained data are shown in Table 1.
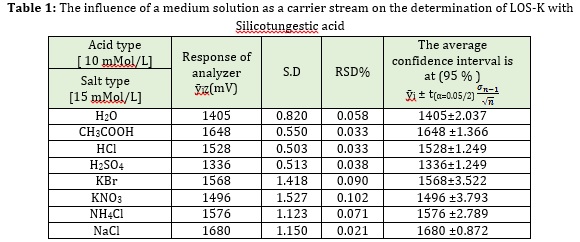
Effect of NaCl on LOS-K–Silicotungestic acid precipitation system
We used previously achieved experimental parameters: LOS-K (2 mMol/L) - Silicotungestic acid (0.5 mMol/L) system at 1.6 and 1.4 ml/min flow rate of carrier stream and reagent respectively with 157 μL sample volume. A variable concentration (5, 10, 15, 20 and 25) mMol/L of NaCl salts was used as a carrier stream in the presence of Silicotungestic acid as a precipitating agent. The increase in peak height with an increase of NaCl concentration up to 20 mMol/L was observed. This increase was due to the regular size of particles compared with the wavelength used for measurement, thus there was the increase on the reflection of most of the incident light, while at high concentration of NaCl (more than i.e., 20 mMol/L) led to the decrease of peak response, which might be due to creating large particles passing the light through the intermolecular spaces of the particles. Therefore, 20 mMol/L concentration of NaCl was chosen as an optimum carrier stream to be used for further experiments.
Physical parameters
Flow rate effect
A study was conducted to determine the preferred flow rate in the range of 0.4-4 ml/min for the carrier stream and 0.35-3 ml/min for the reagent, with a sample volume of 157 μL, fixing all other variables constant (2 mMol/L) LOS-K – (0.5 mMol/L) Silicotungestic acid - (20 mMol/L) NaCl system. Figure 4 shows the 1.2 and 1 ml/min for two lines were respectively optimum flow rate. Because of the increased diffusion and dispersion, the peak width (ΔtB) increased while at higher flow rate > 1.2 and 1 ml/min flow rate for carrier stream and reagent respectively was a slightly decrease of peak response due to departure of precipitate particulate from measuring cell at a short time [18]. Therefore, the optimum flow rate was chosen at 1.2 and 1 ml/min for two lines.
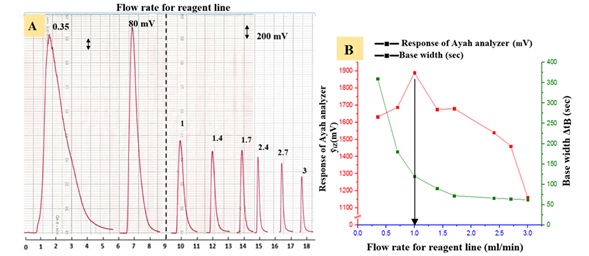
Figure 4: A: Effect of flow rate variation on LOS-K- Silicotungestic acid – NaCl system
B: Relation flow rate variation with recorded response of energy transducer
Effect of variation of sample volume
Variable sample volume's effect (50-250) μL represented 20 cm up to 100 cm Teflon tube of Φ (diameter) 1mm. It was noticed that an increase in sample volume led to increase of peak response up to 157 μL using system of LOS-K (2 mMol/L)- Silicotungestic acid (0.5 mMol/L) – NaCl (20 mMol/L) system at 1.2 and 1ml/min flow rate for carrier stream and reagent, respectively. But, using sample volume more than 157 μL, the decrease of a peak height with increase of base width (ΔtB) was caused by decrease in diverge of light. It was probably due to a slow movement of particles in front of detector, resulting from the continuous relatively longer time duration of carrier stream to pass through injection valve [19]. As a result, 157 μL was chosen as the ideal sample volume to carry out this study.
Purge time
A study was conducted to identify the best injection time, i.e. the maximum time permitted for purging the sample from the injection valve (157 μL sample loop). We used different purge times for the segment at range 5-30 sec and addition open valve. Because the height of the response continued to rise as purge time increased, the open valve time was chosen as the best moment to entirely purge the sample segment from the sample loop. Open valve mode (30 sec) was chosen as the optimum purge time for precipitation system [LOS-K (2 mMol/L)-Silicotungestic acid (0.5 mMol/L)-NaCl (20 mMol/L)].
Reaction coil
Variable coil reaction range (0 - 314) μL was added after the injection valve directly in flow system (between Y-junction and measuring), which allowed mixing the precipitating agent (Silicotungestic acid) with LOS-K; and gave rearrangement of precipitated particulate. The study was achieved at the optimum chemical and physical parameters for previous studies. From figure 5, it was noticed the decrease in the peak high response with an increase of length coil reaction associated with increase of the base width (ΔtB). This was probably due to dilution and dispersion of the segment sample, which reduced the reflected light [20]. Therefore, peak height and clearly response could be seen without coil reaction.
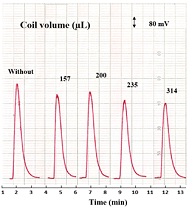
Figure 5: Variation of reaction coil length on: Response profile of precipitation system
Calibration Study of Losartan
The calibration curve of LOS-K was achieved by using the optimum chemical and physical parameters for system. A set of LOS-K solution (0.05- 2.5 mMol/L) was used with Silicotungestic acid – NaCl system at 1.2 ml/min flow rate for reagent. Figure 6.A shows linear calibration curve using a homemade Ayah 6SXl-T-2D- solar cell-analyzer at range (0.05-2 mMol/L) concentration of LOS-K. The new developed methods (Ayah 6SX1-T-2D- solar cell-analyzer) for determination of Losartan potassium were compared with two methods namely turbidimetric method and UV-Spectrophotometric method (classical method), which were based on: UV-Spectrophotometric method (classical method), based on the measurements of absorbance of LOS-K for the range 0.01-0.4 mMol/L at λmax= 232 nm [21]. Figure 6.B shows the best linear range; and Turbidimetric method, based on the reaction of Silicotungestic acid 0.3 mMol/L concentration (already used as optimum concentration after established for range 0.05-0.4 mMol/L Silicotungestic acid) as a precipitating agent with the LOS-K. Figure 6.C shows the best Linear range extended from 0.01-0.5 mMol/L.
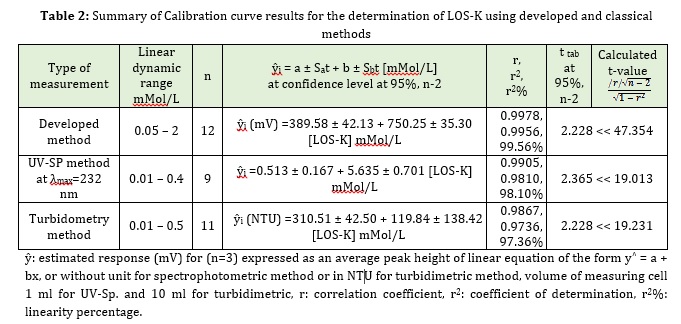
Table 2 summarizes all the results of determination of the LOS-K using a homemade Ayah 6SXl-T-2D- solar cell- analyzer and classical methods (spectrophotometric and turbidometry methods), including: Measurements to estimate value of the response produced from linear regression analysis, linearity percentage, correlation coefficient (r), coefficient of determination (r2) and calculated t-value at 95% confidence level.
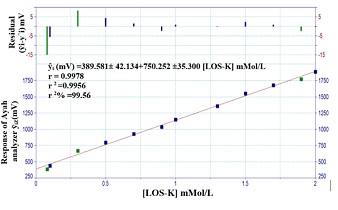
Figure 6.A: Linear calibration curve for determination of LOS-K at range [0.08-2 mMol/L] n=12 by precipitation reaction of LOS-K- Silicotungestic acid (0.5 mMol/L)-NaCl (20 mMol/L) FIA-system
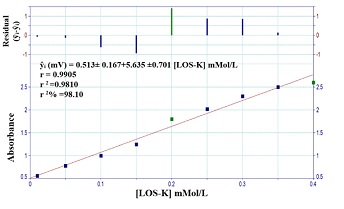
Figure 6.B: Calibration curve for LOS-K using spectrophotometric method at λmax=232 nm
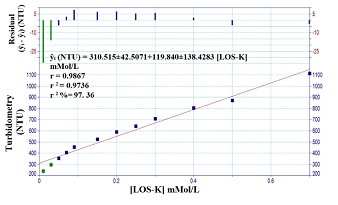
Figure 6.C: Calibration curve for variation of LOS-K concentration using turbidimetric method
Limit of detection
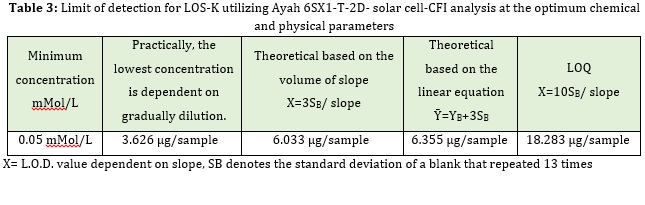
The LOS-K detection limit was found through dilution of the minimum concentration in the linear range with time. Depending on three approaches for determining the L.O.D and LOQ, we achieved limit of detection for LOS-K as shown in Table 3.
Repeatability
The repeatability of the developed method was studied at used concentration of LOS-K (0.1, 0.7mMol/L). Figure 8 shows the repeated measurement of response – time profile in optimum parameters. The repeatability of (% RSD) = 0.5% was found.
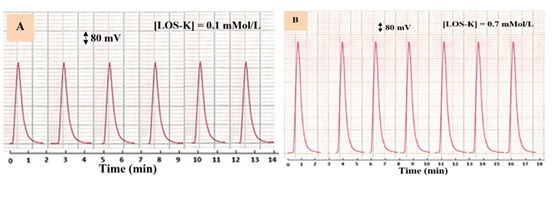
Figure 8: Response profile for six successive repeatable measurements of LOS-K in different concentrations: A-0.1 mMol/L and B- 0.7 mMol/L using LOS-K- Silicotungestic acid– NaCl system
Application
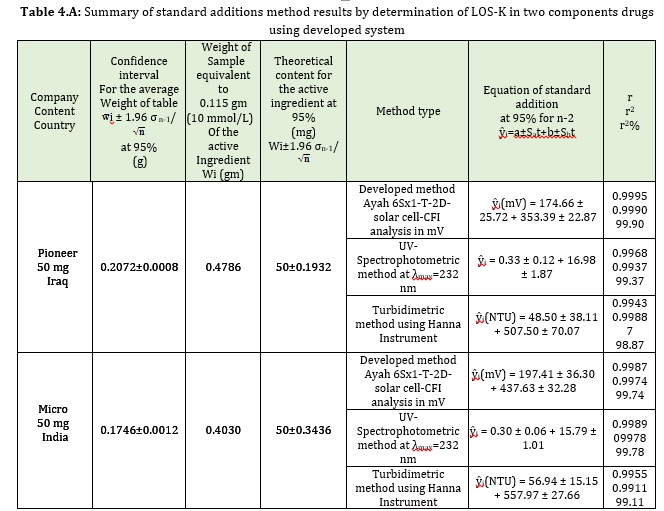
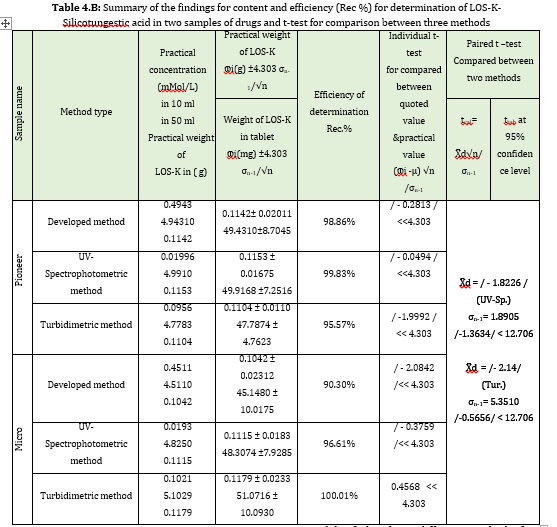
Two different samples of pharmaceutical drug (Iraq, 50mg-pioneer and India, 50mg-Micro) were used to determine the active ingredient of LOS-K. The developed method involves using Silicotungestic acid as reagent (0.5 mMol/L), NaCl (20 mMol/L) system at 1.2, 1 ml/min flow rate for the carrier stream and reagent, respectively. A homemade Ayah 6SX1-T-2D- solar cell was used as detector, which combined with CFI. The results of developed method were compared with classical methods including the measurement of absorbance at λmax= 232 nm by spectrophotometer, while another method involved the measurement of the turbidity of the new reaction of Silicotungestic acid as precipitating reagents with LOS-K to form white precipitate, the response recorded with turbidity meter HANNA(Hungary). Transferring 1 mL (0.5 mMol/L) of each sample to six volumetric flasks yielded a set of solutions from each pharmaceutical medication (10 mL), followed by the addition of 0, 0.3, 0.5, 1, 1.5 and 2 mL from 10 mMol/L of LOS-K standard solution to obtain (0, 0.3, 0.5, 1, 1.5 and 2 mMol/L) and Silicotungestic acid (0.5 mMol/L)-NaCl (20 mMol/L) while in UV-Spectrophotometric method, there was the transferring of 0.04 mL from 5 mMol/L sample solution to each six volumetric flasks (10 mL), followed by the addition 0,0.03 0.05, 0.07, 0.09 and 0.1 mL from standard solution of LOS-K (10 mMol/L) to obtain 0, 0.03, 0.05, 0.07, 0.09 and 0.01 mMol/L; in addition to turbidimetric method, a series of solutions were prepared by transferring 0.2 ml of 5 mMol/L concentration of two samples, followed by the addition of 0, 0.3, 0.4, 0.5, 0.7 and 0. 9 ml from standard solution of LOS-K (10 mMol/L) to obtain 0.3, 0.4, 0.5, 0.7 and 0.9 mMol/L. The results were mathematically treated for standard additions method and tabulated in Table 4.A at confidence interval 95%. The paired t- test calculated for the proposed method and classical methods are showed in Table 4. B. The result showed that there was no significant difference between three methods at the calculated t-value.
Conclusion
Statistical analysis of the results proved high accuracy and precision for the proposed method for losartan potassium analysis. The new method was characterized by speed with high sensitivity. The formulation sample recovery agreed with the acceptable value. The newly developed method was based on precipitation of LOS-K via using precipitate agent (Silicotungestic acid) to form white precipitate, which irradiated by a six-snow white LEDs using Ayah 6SXl-T-2D cell CFI analysis. From the results, it was noticed that three methods of analysis proved to indicate that there was no significant difference between the means (μ) of the three different methods for analyzing the two drugs.
Acknowledgments
The authors extend sincere thanks and appreciation to Prof. Dr. Issam M.A. Shakir and Prof. Dr. Nagam S. Turkey, for their appreciable advice, support, the homemade Ayah 6SXl-T-2D cell analyzer produced by them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
HOW TO CITE THIS ARTICLE
Firas T. Kareem, Mohammad K. Hammood. Determination of the Quantity of Losartan Active Ingredient in the Medication Formulations via Turbidimetric-Flow Injection Technique, Chem. Methodol., 2022, 6(1) 41-51
DOI: 10.22034/chemm.2022.1.4
URL: http://www.chemmethod.com/article_139119.html
- Binh T.T., Tram L.T.P., Hop N.V., Chau N.D.G., Luu N.D., Trang N.T.Q., Anal. Methods Chem., 2021, 2021 [Crossref], [Google Scholar], [Publisher]
- Burnier M., Circulation, 2001, 103:904 [Crossref], [Google Scholar], [Publisher]
- Goa K.L., Wagstaff A.J., Drugs, 1996, 51:820. [Crossref], [Google Scholar], [Publisher]
- Lifshitz I., Kor I., Shabat S., US20060241161A1, 2004 [Google Scholar], [Publisher]
- Lima M.J.A., Reis B.F., Talanta, 2017, 164:183 [Crossref], [Google Scholar], [Publisher]
- Williams R.C., Alasandro M.S., Fasone V.L., Boucher R.J., Edwards J.F., Pharm. Biomed. Anal., 1996, 14:1539 [Crossref], [Google Scholar], [Publisher]
- Latheeshjlal L., Parthiban P., Alagarsamy V., Sunil M., Mahul J.V., Mohan T.R., Chem., 2010, 7:320 [Crossref], [Google Scholar], [Publisher]
- Abdel-Fattah L., Abdel-Aziz L., Gaied M., Spectrochim. Acta A, 2015, 136:178 [Crossref], [Google Scholar], [Publisher]
- Hinge M.A., Bhanusali V.M., Mahida R.J., Chem. Lett., 2016, 6:408 [Crossref], [Google Scholar], [Publisher]
- Sivakumar T., Venkatesan P., Manavalan R., Valliappan K., Indian J. Pharm. Sci., 2007, 69:154 [Crossref], [Google Scholar], [Publisher]
- Baing M.M., Vaidya V.V., Sane R.T., Menon S.N., Dalvi K., Chromatographia, 2006, 64:293 [Crossref], [Google Scholar], [Publisher]
- Bahgat E.A., Innovare J. Med. Sci., 2019, 7:6 [Crossref], [Google Scholar], [Publisher]
- Demirkaya-Miloglu F., Yaman M.E., Kadioglu Y., Brazilian J. Pharm. Sci., 2014, 50:611 [Crossref], [Google Scholar], [Publisher]
- Sharma S., Jadon N., Jain R., Technol., 2018, 5:1 [Crossref], [Google Scholar], [Publisher]
- Issam M.A., Nagam S.T., Ayah 6SX1-ST-2D solar cell CFI Analyzer, Patent, central organization for standardization and quality control- Baghdad-Iraq, GO1N21/00, 2014. [Crossref], [Google Scholar], [Publisher]
- Smith J.G., Organic chemistry, 1st Ed. New York, 2006. [Crossref], [Google Scholar], [Publisher]
- Al-Awadi N.S.T., Aldeen R.A.K., Baghdad Sci. J., 2017, 14:773 [Google Scholar], [Publisher]
- (a) Hammod M.K., development method for determination of metronidazole in pharmaceutical preparation via flow injection technique using phosphotungestic acid as a precipiyant agent, internal Journal of science and research, 2018, 7:409, (b) Jeber J.N., Hassan R.F., Hammood M.K., Al-Jeilawi O.H.R., Actuators B Chem., 2021, 341:130009 [Crossref], [Google Scholar], [Publisher]
- S.T., Al Awadie, O.A. Yassin, Baghdad Sci. J., 2021, 18:565 [Crossref], [Google Scholar], [Publisher]
- Shakir I.M., Hammod M.K., Iraqi J. Sci., 2014, 55:852 [Google Scholar], [Publisher]
- Lastra O.C., Lemus I.G., S ánchez H.J., Pérez R.F., J Pharm Biomed Anal., 2003, 33:175 [Crossref], [Google Scholar], [Publisher]


