Document Type : Original Article
Authors
Department of Chemistry, College of Science for Women, University of Baghdad, Iraq
Abstract
In this work, new complexes of some lanthanide ions metals, such as (La+3, Nd+3, Er+3, Gd+3and Dy+3) with Schiff bases that derived from the antipyrine compound were synthesized. This ligand can be synthesized from condensation dimedone with 4-aminoanitpyrine in microwave irradiation and were identified using (FT-IR spectroscopy, UV-Visible spectroscopy, CHNO analysis, LC-mass, 1H-NMR, 13C-NMR and TGA analysis). The complexes were characterized by FT-IR, UV-Visible, CHNO analysis, conductivity measurement, magnetic susceptibility, and theromgravimatric analysis. From stoichiometry of metal to ligand and all measurements was proposed formula of complexes and a molar ratio (2-2) (metal – ligand) to give the. The bioactivity of the prepared (L) and its complexes were assessed using antibacterial activity, and the results revealed significant activity against some fungi and bacteria.
Graphical Abstract
Keywords
Main Subjects
Introduction
Schiff's bases are one of the most prominent links used in coordination chemistry, as they participate in the preparation of many metal-ion complexes in general and internal transition elements (lanthanides) in particular, due to their ability to coordinate and form complexes. With different combinations and multiple uses [1,2]. In the last century, Schiff is the first to synthesize an imine from a condensation reaction of aldehydes or ketones with primary amines [3,4,5]. Therefore, imines are often referred to by Schiff's bases, and this term has become popular among chemists to refer to organic compounds containing the (-C=N-) group of active azomethine [6- 8].
Schiff's bases and their complexes are of great importance for biological activity [9], these complexes are derived from 4-aminoantipyrine and have been used in various applications in the field of stimulation, and biological activity as anti-inflammatory, bactericidal, fungicide [10, 11] and anticancer [12]. Schiff base ligand contains two groups of donors via nitrogen and oxygen ready to coordinate with lanthanide ions produced a variety of coordination complexes [13], which are of great interest to chemists despite the presence of many Schiff base bonds. Similar studies investigated the coordination links related to their extensive study because they can form easily stable complexes with most metal ions [14].
Lanthanide complexes have attracted widespread interest due to the magnetic and optical properties of these ions, and they can show the coordination number (6-12) with (8 or 9) being the preferred in the complexes [15,16]. Because of the enormous size of ions and their proclivity to form ionic bonds rather than covalent connections that lead to coordination complexes, simple dichroic strands frequently result in complexes with a higher coordination number. Lack of significant crystalline potential of electronic forms 4f, as well as the high ionic radius of these metal ions, which varies dramatically with the atomic number or oxidation number of the lanthanides, contribute to structural variation [17, 18]. Because the 4f electrons in lanthanides are relatively uncoupled in bonding, the fundamental oxidation state of these elements is extremely sensitive to electricity. The ions with a high atomic count have a bigger radius, whereas the ions with a lower atomic count have a smaller radius. The lanthanide contraction can be defined as a parallel slight decrease in the size of the same charge ions with an increase in the nuclear charge between the elements, and the lanthanide expansion can be defined as a parallel slight increase in the size of the same charge ions with an increase in the nuclear charge between the elements [19]. It is also noticed that the great interest in Schiff base complexes is due to the fact that the lanthanide elements bound to Schiff's bases increases potency and activity and thus has a higher biological activity than the ligand due to its paramagnetic properties, therefore it is used in cancer diagnosis and in magnetic resonance imaging [20]. In this research, the aim is to study important structural properties and biological activities of compounds containing 4-amino-antipyrpine. Therefore, we synthesized ligand with its complexes from the lanthanide elements, including (La+3, Nd+3, Er+3, Gd+3 and Dy+3), and studied the biological activity of the compounds against different strains of bacteria [21].
Experimental Part
All chemicals used in this work are of a high degree of purity Supplied by Sigma Aldrich and used by provisioning. The infrared spectrum of (Shimadzu FT-IR 8300) has recorded in the range 4000-400 cm-1, KBr disk in the case of Ligand, complexes was registered with a range of4000-200 nm as a CSI disk on FTIR. UV-visible spectra were measured in DMF solvent using Shimadzu UV-Vis. 160A ultraviolet Spectrophotometer in the range of 190.00-1100 nm. 1H-NMR spectra were recorded in DMSO-d6 on a frequency 400.22 MHz high performance using tetramethyl silicon, all chemical shifts were recorded as ppm, 13C-NMR spectra were recorded in DMSO-d6 on a frequency 100, 64 MHz high performance using tetramethyl silicon, all chemical shifts were recorded as ppm. Mass analysis of ligand has been done with LC-Mass 100P Shimadzu. Magnetic susceptibility for a complex was measured at room temperature using a magnetic susceptibility balance. The conductivity measurement of the complexes in DMF (1×10-3 M) were measured at room temperature using HANNA HI9811-5 conductivity. The melting points of the ligand and all new complexes were determined using a digital melting point scale (Stuart), a model temperature range of ambient to 300 °C.
Synthesis of Schiff’s Bases Method
Preparing a Schiff’s base L was done by mixing in crucible a stoichiometries (1 g) of a dimedone with (2 mol) of 4-aminoanitpyrine and adding 4 drops of glacial acetic acid, the mixture was put in microwave irradiation 170 W for 4 minutes. After completion the reaction, the obtained solid was crystallized by absolute ethanol, some of properties are listed in Table 1.

Scheme 1: Preparation of Ligand
Preparation of Complexes
The complexes were prepared by dissolving (0.1 g) of L, ligand in 5 ml of methanol in a 25 ml round bottom flask, then adding the gradually stirring dissolving (0.16 g) of salts [Ln (NO3)3].x H2O in 5 ml of methanol absolute. A mixture was stirred for a period of 4-7 hours, then left to precipitate. Then precipitate was collected and purified with water and ether and let dry to obtain a pure deposit.

Results and Discussion
IR-Spector of Ligand
Compound [C30H34O2N6] was identified by using FT-IR spectrum, which showed bands at 3437 cm-1, 3116 cm-1, 2951 cm-1, 1651 cm-1, 1621.92 cm-1, 1589 cm-1 and 1523 cm-1, 1315 cm-1 which are attributed to (O-H), due to moisture (22), CH (Aliphatic), CH (aromatic), C=O, C=N, and C=C respectively.
1H-NMR and 13C-NMR Spectrum of Ligand
1H-NMR spectrum of ligand showed a single peak at a chemical displacement to shift 1.02 ppm, it belongs to the proton (H1), while a single peak showed a chemical displacement 2.01 ppm and 3.34 ppm it belongs to the (H2) and (H4) proton in the dimedone compound. The spectrum of ligand showed a single peak at a chemical displacement 3.10 ppm and 4.71 ppm and it belongs to the proton (H3), (H5). While the spectrum showed a triple peak returning to the aromatic ring subordinate to 4-Aminoantipyrine at 7.35 ppm and a binary peak at the chemical displacement 7.54 ppm, while the peak appeared to return to (H6) and (H7) at the chemical displacement. Figure 3 illustrates the ligand 1H-NMR spectrum.
13C-NMR spectra of the Schiff base ligand exhibits signals due to of anitpyrine ring C12 (C=O) carbon at (135.48 ppm), azomethine carbons C13 (C=N) at (153.29 ppm), C1 (10.90 ppm), C2 (28.40 ppm), C3 (32.96 ppm), C4 (36.30 ppm), C5 (39.5 ppm), C6 (40.00 ppm), C7 (40.63 ppm), C8 (50.77 ppm), C9 (124.31 ppm), C10 (126.95 ppm), C11 (129.59 ppm) (Figure 4). Ligand has good solubility in solvent DMSO.
Mass Spectral
LC-mass spectrum is shown in the Figure 2. It was described by the peak of the molecular ions at m / e = 510.57 i.e. the molecular weight m / e was confirmed with a value of 510.56 corresponding to the experimental formula of the linker (C30H34O2N6).

Figure 1: FT-IR of ligand

Figure 2: Ms for ligand

Figure 3: 1H-NMR for ligand

Figure 4: 13C-NMR for ligand
FT-IR spectra of Complexes
The infrared spectrum of the complexes appears as shown in Table 2, where the spectrum of the complexes suffered some slight changes as a result of the coordination of the ligand with ions (La+3, Nd+3, Er+3, Gd+3 and Dy+3), the spectrum gave an absorption peak at a frequency (1583.56 cm-1, 1525.69 cm-1, 1512.19 cm-1, 1558.48 cm-1, 1577.77 cm-1) respectively that belongs to the azomethine group stretching (C=N). This band has a change in the intensity and location of all complex spectra indicating the occurrence of the coordination, and the absorption band of frequency (1620.21 cm-1 - 1651.07 cm-1, 1625.99 cm-1, 1622.13 cm-1, 1660.71 cm-1) respectively, returns to the stretching of the carbonyl group (C=O), and the absorption band at the frequency (426.27 cm-1 , 453.27 cm-1, 452.28 cm-1, 451.43 cm-1, 499.56 cm-1) respectively returns to the stretching of the bond (M-N), and an absorption band at frequency (524.64 cm-1, 520.25 cm-1, 526.57 cm-1, 524.64 cm-1, 524.64 cm-1) respectively back to the stretching band (M-O).


Figure 5: FT-IR of ligand and complexes
Electronic Spectra, Magnetic Moment and Molar Conductance of Complexes
Most lanthanide element complexes display absorption in particular spectrum wavelengths, because most of these complexes are not colored. DMF solvent was used to record the electronic spectra of the produced compounds in the region (200–1100 nm). The lanthanide ions absorb in the visible and the near ultraviolet region, with the exception of the La+3 due to the absence of electrons in the (4f) orbits and the filling of the (4f) orbits with electrons in the lanthanide ions. It can be said that the colors of these ions are due to the (f→f) electron transfer and peaks. The spectrum is in sharp absorption peaks, and the reason for this is that the orbits are (4f) far from the ligand field. Figure (6) shows the electronic spectrum of the ligand, where the appearance of a pick is observed wavelength 285. Figures (7-11), show the electronic spectrum of the complexes and present the synthesized compounds' electronic spectrum, magnetic moments, and Molar Conductance data. The magnetic susceptibility measurements provided magnetic moment values that corresponded to the predicted form.


Thermo Gravimetric Analyses
Thermal significance of Ln+3 complexes from ambient temperature to 700 °C under inert N2 atmospheric conditions by TGA techniques. Complexes Ln+3 of the corresponding associations (Ligand). They were selected for thermal study, and TGA data are listed in Table 4 which shows the TGA curves in the supporting information as shown in Figure 12. The Ln+3 complexes were thermally hydrolyzed in four successive decomposition steps.

Scheme 2: Decomposition steps

Antibacterial Activity
The results of the study proved the biological effectiveness of the preparations ligand and its compounds, as the experiment was conducted under aerobic conditions at a temperature of 37 ° C. Four types of pathogenic bacteria (two types negative for Gram stain and two positive for bacteria stain), using DMF solvent at a concentration of (1 × 10-3 M), were exposed all isolates Pathogenic bacteria of the active compounds by drilling method.
Where the results of the compounds showed their different efficacy against negative and positive staining bacteria, while the Dy+3 complexes did not show any inhibitory activity against the laboratory bacterial species [23]. The data are shown in Table 5.

Figure 12: TGA curve of ligand and lanthanide complexes in under inert N2 atmosphere

The ligand shows a variable activity and the complexes are more active as anti-bacterial, where the ligand appears more active against gram-negative bacteria compared to gram-positive bacteria. Heterogeneous activity was also found in the complexes where some complexes showed their activity against negative bacteria and some against positive bacteria as shown in the table where it shows the Nd+3 complexes are the most effective against all types of bacteria, followed by La+3 and Er+3, while Gd+3 showed its effect only on one type of gram positive bacteria, or Dy+3 did not show any inhibitory effect on all types of bacteria.

Figure 13: the effect of the ligand and its prepared complexes on the growth of bacteria

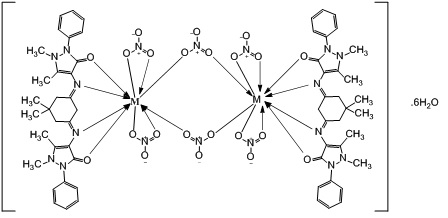
Figure 14: suggested structure for complexes Ln+3=La+3, Nd+3, Er+3, Gd+3 and Dy+3
Conclusion
From the results obtained, it follows that Schiff bases were prepared from 4-aminoantipyrine four-tooth, which were reacted with nitrate lanthanides to produce lanthanide compounds. These complexes were characterized using spectroscopic studies, elemental analyzes, molar conductivity and magnetic moment measurements at room temperature. The compounds were crystalline complexes and were decomposed when heated at N2 to 700 K in four steps. In addition, ligand had no effect on the 4F electrons of the lanthanide ions. The lanthanide ions were coordinated with four nitrogen atoms, four oxygen atoms from imine (azomethine), and 12 oxygen atoms from nitrate. Their biological activity was verified, as well.
Acknowledgments
I would like to express my deepest gratitude to Prof. Dr. Issam M.A. Shakir Al-Hashimi for his appreciable advice, important comments, support and encouragement.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID
Naser Shaalan:
https://www.orcid.org/0000-0002-2875-5056
HOW TO CITE THIS ARTICLE
Kawther Adeeb Hussein, Naser Shaalan. Synthesis, Spectroscopy and Biological Activities Studies for New Complexes of Some Lanthanide Metals with Schiff's Bases Derived from Dimedone with 4-Aminoanitpyrine, Chem. Methodol., 2022, 6(2) 103-113
DOI: 10.22034/chemm.2022.2.3
- Abid K.K., J. Appl. Chem., 2013, 1:87 [Google scholar]
- Sienkiewicz-Gromiuk J., Rusinek I., Kurach Ł., Rzączyńska Z., Therm. Anal. Calorim., 2016, 126:327 [Crossref], [Google scholar], [Publisher]
- Abdulhadi S.L., Abdulkadir M.Q., Al-Mudhafar M.M., Al-Mustansiriyah Journal of Science, 2020, 31:46 [Crossref], [Google scholar], [Publisher]
- Shaalan N., Abed A.Y., Hameed A., Mahde S., J. Chem. Environ, 2019, 23:181 [Google scholar], [Publisher]
- Xu Y., Shi Y., Lei F., Dai L., Polym., 2020, 230:115671 [Crossref], [Google scholar], [Publisher]
- Saleem M.F., Khan M.A., Ahmad I., Aslam N., Khurshid U., J. Pharm. Res., 2021, 20:145 [Google scholar], [Publisher]
- Da Silva C.M., da Silva D.L., Modolo L.V., Alves R.B., de Resende M.A., Martins C.V., de Fátima Â., Adv. Res., 2011, 2:1 [Crossref], [Google scholar], [Publisher]
- Hadi Kadhim S., Abd-Alla Q.I., Jawad Hashim T., J. Chem. Sci., 2017, 15:107 [Google scholar], [Publisher]
- Deshmukh P., Soni P.K., Kankoriya A., Halve A.K., Dixit R., J. Pharm. Sci. Rev. Res., 2015, 34:162 [Google scholar]
- Alam M.S., Lee D.U., Mol. Struct., 2021, 1227:129512 [Crossref], [Google scholar], [Publisher]
- Mangaiyarkkarasi P., Arulantony S., J. Curr. Pharm. Res., 2016, 8:43 [Google scholar]
- Eissa H., Shaalan N., Mahde S., Med. Chem. Inter. J., 2018, 5:55 [Crossref], [Google scholar], [Publisher]
- Shoaib K., Rehman W., Mohammad B., Ali S., Proteom. Bioinform., 2013, 6:153 [Crossref], [Google scholar], [Publisher]
- Halli M.B., Sumathi R.B., J. Chem., 2017, 10:S1748 [Crossref], [Google scholar], [Publisher]
- Naik V.M., Mallur N.B., Orbital: Electron. J. Chem., 2011, 8:1900 [Crossref], [Google scholar], [Publisher]
- da Rosa P.P.F., Kitagawa Y., Hasegawa Y., Chem. Rev., 2020, 406:213153 [Crossref], [Google scholar], [Publisher]
- Abbas A.K., Iraqi J. Sci., 2015, 56:3297 [Google scholar], [Publisher]
- Sobolev B.P., Rep., 2020, 65:175 [Crossref], [Google scholar], [Publisher]
- Silva G.S., Dutra J.D.L., da Costa N.B., Alves Jr. S., Freire R.O., Phys. Chem. A, 2020, 124:7678 [Crossref], [Google scholar], [Publisher]
- Ahmed R.M., Yousif E.I., Hasan H.A., Al-Jeboori M.J., World J., 2013, 2013. [Crossref], [Google scholar], [Publisher]
- Hussain Sumrra S., Imran M., Ibrahim M., Ambreen S., Mehmood R., Assiri M.A., Irfan A., Chil. Chem. Soc., 2021, 66:5057 [Crossref], [Google scholar], [Publisher]
- Hassan S.A., Lateef S.M., Majeed I.Y., J. Pharm. Technol., 2020, 13:3001 [Crossref], [Google scholar], [Publisher]
- Hammer A., Heinemann D., Hoyer C., Kuhlemann R., Lorenz E., Müller R., Beyer H.G., Remote Sens. Environ., 2003, 86:423 [Crossref], [Google scholar], [Publisher]