Document Type : Original Article
Authors
Department of Chemistry, College of Science for Women, University of Baghdad, Baghdad, Iraq
Abstract
This study discusses the preparation of new series of tetra-dentate complexes Cu (II), Ni (II), Co (II), Zn (II), and Cr (III) complexes with Schiff base (ligand tetradentate) derived from isatin with 2-aminobenzohydrazide, characterized by IR spectroscopy, mass spectra, UV-Vis, 1H-NMR and elemental microanalysis. The complexes Cu (II), Ni (II), Co (II), Zn (II), and Cr (III) were characterized using IR spectroscopy, UVVis, magnetic susceptibility, conductive, elemental microanalysis and metal analysis by atomic absorption. The proposed structures of complexes from the all measurements showed that Ni (II), Co (II), Zn (II), and Cr (III) have octahedral shape and Cu (II) has square planer shape. Bioactivity of the Schiff base and complexes were assessed examined with antibacterial activity. Studies were conducted to assess the growth-inhibiting potential of the complexes synthesized, and the ligands, against various bacterial strains.
Graphical Abstract
Keywords
Main Subjects
Introduction
Heterocyclic compounds are cyclic organic compounds that contain at least one hetero atom. The most common hetero atoms are oxygen, sulfur, and nitrogen. However, hetero cyclic rings contain other hetero atoms are known [1-2]. Hugo Schiff (1864) first identified Schiff base [3,4] as a condensation reaction between aldehyde or ketone with primary amine [5]. (imine, anils and azomethine) are other names for Schiff base [6]. Schiff's foundation is one-of-a-kind and efficient multi-dentate ligand. Schiff bases are extensively studied in coordination chemistry, particularly in compounds containing heterocyclic compounds with the imine group [7], because they have basic properties due to the presence of a single electron pair on the imine nitrogen atom (C=N) [8], and they are frequently pentagonal or hexagonal rings with the transition metal ion [9]. The Schiff base result of 2-aminobenzohydrazide by functionalizing the amino group with heterocyclic compounds has inhibitors for anti-inflammatory, anti-fungal, and antibacterial agents with a broad pharmacological range [10,11]. Complexes of transition metal ions with Schiff bases heterocyclic compounds were characterized using various spectral methods in this study.
Methodology
Materials
All chemicals used for the experimental work were sourced from (Sigma-Aldrich) such as, 2- aminobenzohydrazide 97%, Isatin 97%, NiCl2.6H2O 99%, CuCl2.2H2O 99%, CoCl2.6H2O 99%, ZnCl2 and CrCl3.6H2O 98%.
Instrumentation
IR-spectra of ligand and complexes were obtained in the range (4000-400) cm-1 (as well as KBr discs) were recorded by Shimadzu spectrometer. Shimadzu spectrometer UV-1800 was used to measure the spectra for the ligand and the synthesis complexes. Mass Spectroscopy of the ligand were recorded by a conductivity meter model Johnson Matthey Catalytic System Division, England product was used to calculate conductivity. NMR-(SMSO-499.44Hz). CHN analysis was done by using analyzer model 5500 Carlo-Erba.
Preparation of ligand
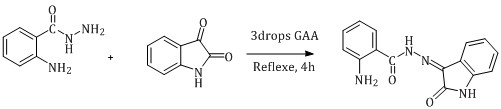
Two solutions were prepared using a round-bottom flask, solution A and solution B. For solution A, (0.006 mol) isatin dissolved in (10 mL) of absolute ethanol and for solution B, (0.006 mol) 2-aminobenzohydrazide dissolved in (10 mL) of absolute ethanol. Next, solution A was deposited dropwise onto solution B under stirring and glacial acetic acid was used as a catalyst. The mixture was reflexed with constant stirring for (4 h). After that, the precipitate was filtered, washed with ethanol, and left to be dried. The melting point (m.p.) yield, and C.H.N.O analysis are listed in (Scheme 1).

Scheme 1: Synthesis of (L) ligand
Preparation of Metal Complexes
A methanol solution of the ligand (0.006 mol) of the ligand dissolved in (10mL) of methanol in around-bottom flask, then we added dropwise (0.006 mol) from the solution of metal ion Cu (II), Ni (II), Co (II), Zn (II) and Cr (III), chloride dissolved in (10mL) of methanol. The mixture has been refluxe with constant stirring for (2-3 h), the solution cooled, then the precipitate was filtered, washed with ether. The properties of their complexes are shown in Table 1.

Result and Discussion
Spectra of ligand
The passing of the carbonyl group of the Isatin in the region (1732) cm-1 and the amino group of the 2-aminobenzohydrazide in the region (3337-3317) cm-1 was shown in the FT-IR-spectra of the synthesized ligand, as well as the occurrence of a new beam of the synthesized ligand in the region (1616) cm-1, which belongs to the imine group [12]. Figure 1 and Table 2 present the FT-IR-Spectra for the synthesized ligand and its complexes.
1H-NMR Spectra.
1H-NMR-(SMSO-499.44Hz): δ=13.86 (s, 1H, NH), 11,34 (s, H-NH amide) ,10.81 (s, 2H, NH2), 6.63-7.9 (m, 4H, H-Ar). Figure 2 see 1HNMR for ligand.
Mass Spectra.
The molecular weight of the synthesized ligand was measured by mass spectra. The mass spectra of the ligand are given a peak at m/es=280.1 that is consistent with the theoretical value of 280.28. Figure (3) shows the mass spectra.

Figure 1: FT-IR for Ligand

Figure 2: 1H-NMR for Ligand

Figure 3: mass spectra of ligand
(FT-IR) spectra of complexes
The imine group (C=N) amplitude frequency in the ligand appeared in the region (1616 cm-1) when connected to the nitrogen atom. These stretchy frequencies in all complexes shifted to lower frequencies that differ from those in the ligand, indicating that (C=N) is involved in the coordination with the metal, which is consistent with the aforementioned research on Schiff base [13]. The (–NH-C=O) moiety presence at (1650 cm-1) in ligand shifted to the lower frequencies in complexes [14]. The infrared spectrum of the prepared complexes revealed a stretching of group (M―N) confirming in the bound region between (447–489 cm-1) [15], supporting the metals association with Schiff base through the nitrogen atom of the imine group (C=N), and suggesting the metals binding in the prepared complexes. A new beam emerged in most of the complexes in the range (515-582 cm-1) revealed a stretching of group (M―O) [15], suggesting that the metal in these complexes is bound to the oxygen atom in the ligand.

Electronic Spectra
The electron spectrum results of the prepared ligand shows that it dissolved in DMF. The main bands resulting from electronic transmission (p→p*) in the range (264 nm) were shown, related to the aromatic ring. Furthermore, another package in range (399 nm) related to electronic transmission (n→p*) belongs to C = N, C = O (17). In the spectra of the prepared complexes, these beams are shifted towards different frequencies [18], indicating a symmetry between the ligand and the metal ion (19). All electronic transmission shown in Table 3.


Figure 4: UV-visible spectra for ligand and complexes

Figure 5: Suggested structure for complexes
Biological activity
The drilling method experiment was carried out, and four types of pathogenic bacteria were developed (Staphylococcus aurius, Escherichia coli, Bacillus subtitles, and Klebsiella pneumonia) under aerobic conditions at (37C°) for (24h). The compound were effective against positives to stain bacteria (two positives for Gram stain, and two negatives for Gram stain [20]. The ligand and its complexes (L1 and L2) shown more activeness towards Staphylococcus aurius than other bacteria, L3 shown more activity towards Bacillus subtitles than other bacteria. While, L4 and L5 shows more activeness towards Klebsiella pneumonia than other complexes. The pictures below show the effect of the ligand and its complexes on bacteria.


Figure 6: Antibacterial activity of Schiff base and complexes
Conclusion
Throughout our study of the physical properties and by performing different types of analysis such as (FT-IR, UV-VIS Spectra, mass spectra and conductivity) of the prepared ligand and its complexes, we suggested that, Zn(II), Ni(II), Co(II), Cr(III) have octahedral geometry and have a general formula [MLCl2], Cr(III) has octahedral geometry and have a general formula [MLCl2] Cl, Cu(II) has square planer geometry and has a general formula [ML]Cl2.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID
Naser Shaalan:
https://www.orcid.org/0000-0002-2875-5056
HOW TO CITE THIS ARTICLE
Luma Hameed, Naser Shaalan, Synthesis, Characterization and Biological Activity Study of some New Metal Ions Complexes with Schiff’s Bases Derived from Isatin With 2-Aminobenzohydrazide. Chem. Methodol., 2022, 6(2) 137-145
DOI: 10.22034/chemm.2022.2.6
- Carey A.F., Giuliano M., Organic Chemistry 8th ed, N.Y: McGraw-Hill, 2014, 128 [Publisher]
- Al-Mulla A., Der Pharma Chem., 2017, 9:141 [Google scholar], [Publisher]
- Shaalan N.D., Abdulwahhab S.M., J. Chem., 2021, 64:4059 [Crossref], [Google scholar], [Publisher]
- Da Silva C.M., da Silva D.L., Modolo L.V., Alves R.B., de Resende M.A., Martins C.V., de Fátima Â., Adv. Res., 2011, 2:1 [Crossref], [Google scholar], [Publisher]
- Abu-Dief A.M., Mohamed I.A., Basic Appl. Sci., 2015, 4:119 [Google scholar]
- Gosh P., Dey S., Aral M., J. Chem., 2019, 62:523 [Crossref], [Google scholar], [Publisher]
- Ismael M., Hmood A.B., Shaalan N., Al-Taay W.A., Hasan A., Ali M., Al Ahmed A., Yousif E., J. Pharm. Biol. Chem. Sci., 2016, 7:2347 [Google scholar]
- McCarty C.G., The Chemistry of the Carbon-Nitrogen Double Bond, Wiley-Interscience, New York, 1970 [Google scholar], [Publisher]
- Shaalan N., Abed A.Y., Alkubaisi H.M., Mahde S., J. Chem. Environ., 2019, 23:181 [Google scholar], [Publisher]
- El-Shaieb K.M., Ameen M.A., Abdel-Latif F.F., Naturforsch. B., 2012, 67:1144 [Crossref], [Google scholar], [Publisher]
- Arjun H.A., Elancheran R., Manikandan N., Lakshmithendral K., Chem., 2018, 7:1 [Crossref], [Google scholar], [Publisher]
- Islam M.R., Mohsin M., Bangladesh J. pharmacol., 2008, 2:7 [Crossref], [Google scholar], [Publisher]
- Sridharand S.K., Ramesh A., Pharm. Bull., 2001, 24:1149 [Crossref], [Google scholar], [Publisher]
- Pousaneh E., Sadighian S., Bikas R., Hosseini-Monfared H., Sousaraei A., Siczek M., Lis T., Mol Struct., 2020, 1199:127023 [Crossref], [Google scholar], [Publisher]
- Andiappan K., Sanmugam A., Deivanayagam E., Karuppasamy K., J. Biol. Macromol, 2019, 124:403 [Crossref], [Google scholar], [Publisher]
- Abd El‐Halim H.F., Mohamed G.G., Anwar M.N., Organometal. Chem., 2018, 32 [Crossref], [Google scholar], [Publisher]
- Sangeeta S., Ahmad K., Noorussabah N., Bharti S., Mishra M.K., Sharma S.R., Mol Struct., 2018, 1156:1 [Crossref], [Google scholar], [Publisher]
- Haribabu J., Alajrawy O., Jeyalakshmi K., Balachandran C., Acta A Mol Biomol, 2021, 246:118963 [Crossref], [Google scholar], [Publisher]
- Zeyrek C.T., Elmali A., Elerman Y., Svoboda I., Naturforsch. B, 2005, 60:143 [Crossref], [Google scholar], [Publisher]
- Kareem S., Shaalan N.D., Methodol., 2021, 6:1 [Crossref], [Google scholar], [Publisher]