Document Type : Original Article
Authors
1 Department of Chemistry, Tabriz Branch, Islamic Azad University, Tabriz, Iran
2 Biotechnology and Medicinal Plants Research Center, Ilam University of Medical Sciences, Ilam, Iran
3 Department of Pharmacology, Medical School, Ilam University of Medical Sciences, Ilam, Iran
4 Department of Computer Engineering, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
5 Department of Endodontics, Dental School, Ilam University of Medical Sciences, Ilam, Iran
Abstract
The aim of the study was to produce a Polyvinyl chloride-based membrane containing a polymer imprinted with a valproic acid molecule which was directly coated on a graphite electrode to determine the amount of valproic acid in aqueous samples. With the dispersion of molecularly imprinted polymer particles in dioctyl phthalate plasticizer as a solvent mediator, this potentiometric sensor was designed and after that it was embedded in a polyvinyl chloride matrix. The designed electrode showed a near-Nernstian slope of 54.1 ± 1 mV decade-1 in the concentration range of 10-6-10-2 M and a detection limit of 10-6 M with a response time of about 40 seconds for valproic acid and can be used for 2 months without divergence changing the potential. The fabricated electrode, in the pH range among 3.5-8.5, indicates the good sensitivity to valproic acid relative to the other ions. Finally, this electrode can be used as an indicator electrode in determining the concentration of valproic acid in valproic acid tablets.
Graphical Abstract
Keywords
Main Subjects
XIntroduction
Valproic acid (VPA) (epilepsy, anticonvulsant, and mood stabilizer drug) is a fatty acid with a short-chain which is a good histone deacetylase (HDAC) inhibitor [1,2]. Due to the minimally cytotoxic and biological relevance of VPA, monitoring drug levels in various matrices is valuable (particularly in epilepsy) for the management of therapeutic drug efficiency [3]. There are several methods for VPA determination including High-performance liquid chromatography (HPLC) with ultraviolet (UV) detection [4], fluorescence detection [5] or coupled with mass spectrometry (MS) [6], capillary electrophoresis (CE) [7], and gas chromatography (GC) [8]. VPA analysis in biological samples is difficult due to the complicated matrix (presence of organic compounds, proteins, and salts). Hence, sample preparation (analyte pre-concentration and sample cleanup) is crucial in drug analysis [9]. The most important problems in using chromatographic methods are the need for derivatization and time-consuming methods. Because the use of techniques requires precision tools and operating costs, the development of faster, simpler, and less costly but still sensitive electrochemical techniques can be an interesting option.
Molecularly imprinted polymers (MIPs) are synthetic materials which have a large number of diagnostic cavities that are complementary to the target molecule (i.e., template molecule) in size, shape, and functional group. Therefore, MIPs demonstrated high detection power and excellent binding affinity for the target molecules and are known as synthetic antibodies [10,11]. Recently, MIPs have been used as selective solid adsorbents in various fields such as separation, catalysis, manufacturing of sensors, preconcentration, chromatography stationary phases, immunoassay, and others [12,13].
There are only a few research reported valproic acid as a template in the field of HDAC inhibitor molecularly imprinted polymers [14], and also, few sources are concerned directly with valproic acid in the field of electrochemical methods [15, 16].
This study aims to construct a potentiometric sensor using a MIP-based membrane and its usage in the determination of valproic acid in aqueous solutions. Due to their improved mechanics, the use of ion-selective electrodes has been widely common in the production of potentiometric sensors [17]. The advantage of potentiometry over other methods of analysis is its economic cost which is fairly cheap. Potentiometric sensors, compared to the other decomposition methods, only slightly perturb the sample and do not require pre-preparation methods and application of agents [18]. In this work, a MIP-based ion-selective electrode for valproic acid in tablets by the casting of membranes after dispersion prepared imprinted polymer in dioctyl phthalate (DOP) and embedded in polyvinyl chloride (PVC) matrix.
Materials and methods
All reagents were analytically equivalent and for preparing the solutions, double-distilled water was used. The valproic acid tablet was prepared from a local pharmacy in Ilam, Iran (Abidi. Co). Valproic acid powder, 3-aminopropyl triethoxysilane (APS), silica gel, tetra ethoxy silane (TEOS), acetic acid, methanol, hydrochloric acid, salts of all cations used, and sodium chloride (Merck), Sodium tetraphenylborate, dioctyl phthalate (DOP), dibutyl phthalate (DBP), polyvinyl chloride (PVC), and oleic acid (OA) (Fluka) were used. A Philips saturated calomel reference electrode (SCE) vs. a digital Hioki 3200 multimeter were measured potentials (Lab Junction Potentiometer with Stirrer (Digital), Model: LJ-118/S). FTIR spectra were recorded by the FTIR spectrometer (Shimadzu, F8400, Japan) with the pharmacopeia method [19]. A pH meter (Metrohm Swiss-made, 211) was used for pH adjustments.
Valproic acid imprinted silica gel adsorbent preparation
The synthesis procedure for MIP was as follows:
Briefly, the activation of silica gel surfaces was done by reflux with HCL under stirring, then filtered and washed by distilled water several times and dried, as well [20]. Next by dissolving valproic acid in methanol (0.5 ml,10–2 M) and adding APS (2.5 ml), TEOS (5 ml,10 min stirring), and then acetic acid (1.5 ml, 1 M), imprinted silica gel adsorbent was prepared. After washing and drying the powder, it was stirred with methanol and HCL solution for 3 h, and finally to neutralize the precipitate, it was washed 5-6 times with sodium hydroxide [21,22].
Electrode Preparation
The PVC membrane preparation was carried out according to the reported procedure [23,24]. First, PVC (60 mg) was dissolved in THF (2.5 ml). Then, MIP (100 mg) was dispersed in DOP (0.45 ml, as solvent) which was added to the solution and homogenized. Next, 1g of sodium tetraphenylborate was added as an additive. Graphite electrodes (10 mm long and 3 mm diameter, Rotring HB) were prepared and the electrode was polished with sandpaper, boiled in nitric acid, then after washed with distilled water, and dried in the oven. They were then immersed in a solution of tetrahydrofuran (THF) and heated under a hood until THF completely evaporated. The ends of the graphites dipped into the membrane solution several times to form a 1 mm thick membrane. The membrane is immersed in the prepared solutions of valproic acid (10-4 M) for 20 hours.
A measurement model and EMF Measurements for the performance of the membrane
After preparing the membrane, drawing a calibration curve to convert the obtained potential values to the concentration of valproic acid in various experiments is necessary. For this purpose, six solutions with different concentrations of 10-2, 10-3, 10-4, 10-5, 10-6, 10-7M were prepared and their potentials were read. The calibration curve is presented using Excel diagrams.
The cells’ assemble which have been used to measure all EMFs were as follows:
Graphite electrode|MIP-based membrane|Sample solution||SCE
The prepared MIP electrode (as measuring electrode) was used along with the SCE and from the Debye-Huckel equation, the activity coefficients were calculated:
In which, γ, μ, and z are the activity coefficient, solution ionic strength, and charge of the ions, respectively.
Results and Discussion
Preparation and characterization of MIP
Surface molecularly imprinted polymers are synthesized in rigid matrices through the process of combining template molecules and have less functional monomers than acrylic-based polymers. In these structures, both APS and TEOS are mainly used and the advantages of sol-gel MIPs are mild reaction conditions, easier operation, and use of their high surface area [25,26].
It should be noted that in many non-covalent sol-gel MIPs preparations, the pattern of the template: monomer: cross-linker with a molar ratio of 1: 6: 1, has led to their proper performance [27]. So, in this work, the same ratio was used for the synthesis of valproic acid-surfaced MIP. One of the most common functional monomers is 3-aminopropyl triethoxysilane (APS), which in addition to its strong ionic bonding property can interact with acidic groups [28]. Since the morphology and chemical environment of the MIP are greatly influenced by the choice of cross-linking monomer, careful selection is important. TEOS, as a cross-linker, determines the thickness of the MIP layer [29]. To use solvents following green chemistry and to prevent the use of halogenated solvents, acetic acid was selected as the solvent, which prevents the formation of hydrogen bonds between valproic acid and the functional monomer and also leads to easier valproic acid removal [30]. Accordingly, for the synthesis of surfaced MIP, APS, TEOS, and acetic acid were used as functional monomers, cross-linker, and solvent, respectively. The obtained surfaced-MIP was characterized by FT-IR.
FT-IR spectra were obtained from non-imprinted and valproic acid-imprinted silica gel adsorbents (Figure 1) which provided enough evidence for the successful MIPs preparation. As displayed in the following Figure, VPA is an aliphatic carboxylic acid having a strong absorption peak in the carbonyl group in the 1700-1710 cm -1 and a broad hydroxide absorption peak in the 2400-3400 cm- 1.
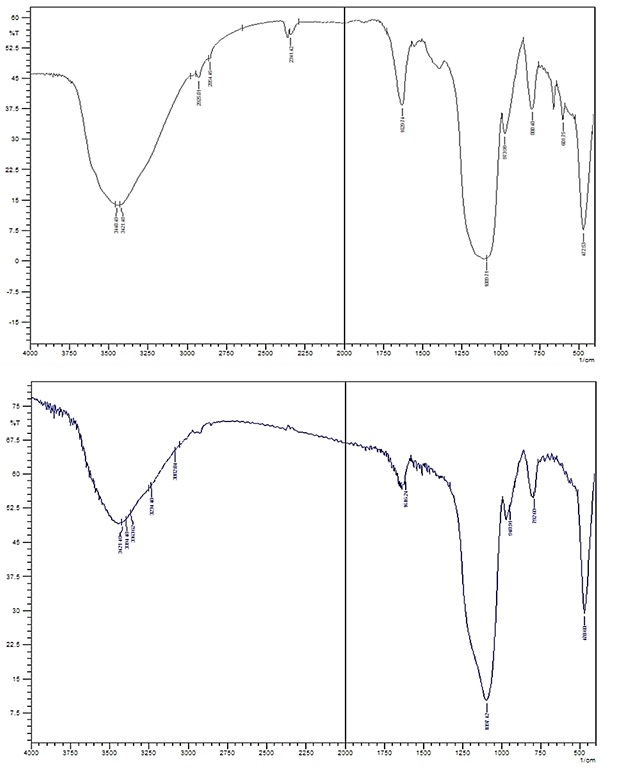
Figure 1: FT-IR spectra of silica gel before and after imprinting
Optimization of membrane composition
In chemical and biosensor sensors, the analyte binds to the diagnostic elements and produces a physical or chemical signal. A processor then converts the signals into measurable ones. If MIP is used instead of the biological molecule as the recognition element, the same general principle is still applied [31]. Successful production of selective electrodes with MIPs for multiple drugs and herbicides opened new horizons for sensor technology [32-35]. Since MIPs have binding sites which complement the template molecule in shape and size, it can be mentioned that the polymer has a molecular memory for analyte reselection. In this research, the optimization of conditions has been investigated for better valproic acid-selective electrodes based on MIP membrane performance. It has been indicated that the sensitivity and selectivity of ion-selective electrodes are significantly affected by important properties of PVC membranes such as solvent (plasticizer), nature, and amount of ion recognizing material, and in particular, the additives nature [36,37]. Thus, different parameters influenced on the synthesized imprinted polymer membrane were optimized and the results are summarized in Table 1.
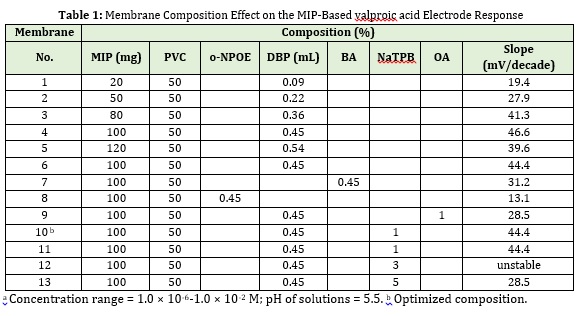
As depicted in Table 1, the potential slope maximized with increasing MIP from 20 to 100 mg, indicating an increase in membrane efficiency. However, with increasing the amount of polymer to 120 mg, the potential slope is reduced, which illustrates a decrease in membrane efficiency. It is obvious that increasing the amount of polymer due to the increase of active adsorption sites increases the efficiency of the membrane, however increasing the amount of polymer due to the imbalance of the ratio of polymer and emollient, reduces sensitivity and efficiency [38].
By changing the type of plasticizer to dibutyl phthalate DOP, the potential slope has increased, which indicates the increase in membrane efficiency. The plasticizer or solvent nature of the membrane, due to its high weight percentage in the membrane composition, strongly influences its electrochemical properties, which include potentiometric sensitivity, and is generally expected to be similar to the membrane dielectric constant similar to the liquid softener dielectric constant. Among the three different plasticizers used, the membrane prepared with DOP had the best performance and the best response. DOP has a dielectric constant of 6.4, which is higher than the dielectric constant of benzyl acetate (BA), which is 1.5, which increases the slope of its potential line, but ortho-nitrophenylectyl ether (o-NPOE) reduces the slope of the reduction potential. This compound has more polarity than DOP (23.9), but both are equally lipophilic. DOP seems to perform better due to its polarity-lipid balance. The best detection limit was obtained when these characteristics reached the mean value [39].
By changing the additive type from oleic acid to sodium tetraphenylborate, a potential slope has been raised which indicates an increase in membrane efficiency. Adding cationic or anionic additives to the membrane increases both the strength especially in high voltage applications [40] and the charge carrying capacity of the ion-selective electrodes, and this is consistent with finding natural carriers for the electrode. Electrodes with no-charge sites respond to cationic additives because the polyvinyl chloride on the market contains some anionic impurities which act as anionic sites. The sensitivity of polyvinyl chloride membranes is very low in the absence of lipophilic cationic additives. However, the presence of the additive increases the sensitivity and decreases the involvement of the sample anions, and changes the membrane behavior, as well. As depicted in Table 1, under the same conditions, the sodium tetraphenylborate additive performed much better than the oleic acid additive. Studies have revealed that under similar conditions, a 10% increase in oleic acid has similar results to a 2% w/w increase in sodium tetraphenylborate [39, 41].
By increasing the amount of additive from 1 mg to 3 and 5 mg of sodium tetraphenylborate, the potential slope was reduced, which indicated a decrease in membrane efficiency. It can be due to its undesirable load transfer mechanism or disturbance of the weight-weight ratio of membrane components and its deviation from the Nernstian behavior. Likewise, increasing the amount of additive not only does not have a favorable effect on the electrode response but also makes the potential very unstable [42].
Conditioning Solution Concentration
The membrane electrode was investigated with different concentrations of conditioning solution from 1.0× 10-2 M to 1.0 × 10-7 M. As illustrated in potential-log C valproic acid plots, the slope in the solution of 10-4 M of valproic acid has the highest value compared to the solutions of 10-3 M and 10-5 M, which indicates the high efficiency of the membrane. The low efficiency of the membrane prepared with the prepared solution of 10 -5M is since the concentration of the drug is very low and it is not able to provide the necessary material to fill the existing cavities. Concentration is very high in 10-3 M solution and causes saturation of detection cavities. But 10-4 M solution shows the best performance. The low efficiency of membranes in 10-3 M and 10 -5M solutions can be due to the membrane surface losing its sensitivity due to the interference of disturbing ions or surface damage due to mechanical friction [43].
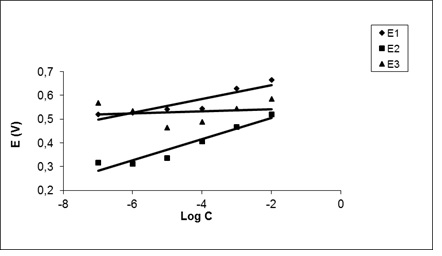
Figure 2: Investigation of the effect of conditioning solutions on membrane performance using potential-log C valproic acid plots; E1: the potential related to valproic acid concentration of 10-3 M; E2: the potential related to valproic acid concentration of 10-4 M; E3: the potential related to valproic acid concentration of 10-5 M
Response Time and Reversibility
Electrode response time is a prominent factor in analytical applications. The practical or mean response time (required time to reach 90% of the maximum potential) was obtained by immersing the sensor in valproic acid solutions with a concentration difference of 10-fold. (Figure 3; two isolated jumps in concentration, 5.0 × 10-6 M to 5.0 × 10-5 M and 5.0 × 10-5 M to 5.0 × 10-4 M). As depicted in the following Figure, the response time of a stable membrane electrode is found to be about 40 seconds. It is reported that the use of electrodes containing solids is often limited due to the poor response time and low stability [44], the response time of this electrode is fast and the lifetime is reasonable to be at least 3 months.
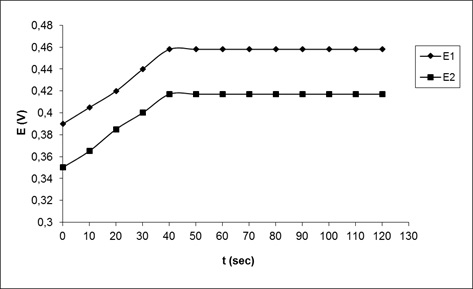
Figure 3: Response time of the valproic acid selective electrode with two isolated jumps in concentration; (1) 5.0 × 10-6 M to 5.0 × 10-5 M, (2) 5.0 × 10-5 M to 5.0 × 10-4 M
Effect of pH
The membrane sensor dependence to pH was investigated in concentrations of 10-4 and 10-5M valproic acid in the range of 2 to 10 (Figure 4). The pH of the solutions was adjusted by adding small amounts of sodium hydroxide and hydrochloric acid. As displayed in the following Figure, the potential in the range of 3.5-8.5 at concentrations of 10-4 and 10-5M M is a pH-independent sample. As the pH of the electrode increases further, it acts irregularly, which may be due to the formation of some hydroxy complexes or deposits from the base of the compound which reduces the potential of the electrode. It seems that at pHs less than 3, the electrode responds to the hydronium ions present in the sample solution, which increases the electrode potential [44].
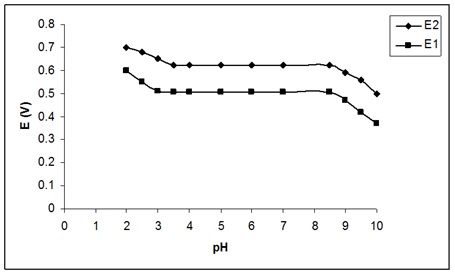
Figure 4: Effect of solution pH on the potential response of the valproic acid selective sensor at (1) 1.0 × 10-4 M and (2) 1.0 × 10-5 M
Evaluation of sensor detection limit by calibration curve
After optimizing the membrane composition, the calibration curve in the concentration range of 10-2 M to 10-7 M was investigated and the results are depicted in Figure 5.
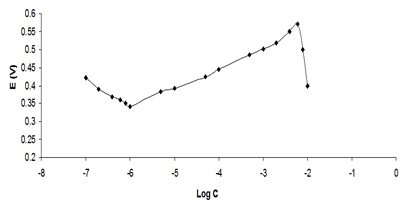
Figure 5: Investigation of the detection limit for the designed membrane using a potential curve in terms of concentration logarithm
As can be visible from the Figure, the electrode has a slope of 0.058 in the range of 10-6M to 4*10-2 M, which is completely compatible with the Nernst slope. The detection limit for this electrode using the standard calibration curve was about 10-6M, which is much lower than the detection limit for membranes based on potentiometric sensors [44].
Potentiometric Selectivity
The selectivity coefficient describes the effect of interfering ions on the response behavior of ion-selective membrane electrodes. The reaction between small molecules or ions with large molecules of biological systems and with special acceptors on the surface of the structure of super molecules is one of the most significant factors in biochemical and biophysical studies. This includes a wide range of important biological factors such as binding to small cations, especially metal ions, proteins, and nucleic acids [45]. This coefficient is obtained from different equations [46,47].
As depicted in Table 2, the electrode was selective to valproic acid compared to the other cations tested, and the selectivity coefficients are about 10-3 or less, indicating that these cations have little effect. They have valproic acid on the sensor function. Potential diagrams - concentration logarithms for valproic acid and disturbing ions are obtained separately using the target electrode system. Then, using a pair of values (aA) which is the initial concentration and (aB) as disturbing concentration, so that the electrodes have the same potential values in separate solutions and the equation
= Ln aA/Ab(2/z) KA,B Pot
In which, z ion-ion charge is disturbing, the selectivity coefficient was measured. The data presented in Table 2 showed that in many cases the selectivity coefficient obtained for the graphite-coated electrode is very low. Graphite thin layers have been considered in electronic applications due to their porous structure and high ion transfer rate [48]. Based on the results, the high selectivity of the designed membrane to valproic acid compared to other metal ions is due to its lipophilicity and high molecular hardness. As expected, the electrode acts well as a receptor for valproic acid [45].

Preliminary applications
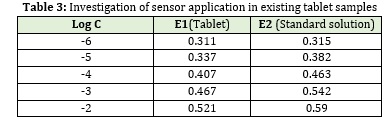
Determination of valproic acid in tablet
The designed membrane electrode can be used to directly measure valproic acid in the tablet. For this, 10 tablets of valproic acid (containing 145 mg of valproic acid, 333 mg of sodium valproate, and the other substances including silica and calcium chloride) were weighed and powdered. An exact weight ratio of powder equivalent to 5 mg of active ingredient was dissolved in 5 ml of sodium hydroxide solution. The solution was completely homogenized and filtered. Then before measurement, the prepared solutions were properly diluted for measurement in the linear range. The membranes are then placed in prepared solutions of 10-4 M valproic acid for 24 hours. Then at specified time intervals, the potential of 10-7-10-2 M solutions of the drug-using prepared membranes was measured.
The selectivity coefficient describes the effect of interfering ions on the response behavior of ion-selective membrane electrodes. The reaction between small molecules or ions with large molecules of biological systems and with special acceptors on the surface of the structure of super molecules is one of the most significant factors in biochemical and biophysical studies. This includes a wide range of important biological factors such as binding to small cations, especially metal ions, proteins, and nucleic acids [45]. This coefficient is obtained from different equations [46,47].
Conclusion
This study proposes a new method for constructing a potentiometric sensor for selective, rapid, and direct detection of valproic acid, which involves dispersing the polymer particles imprinted by valproic acid in dioctyl phthalate, separating the mixture from the silica gel base, and pouring the final composite. The resulting membrane electrode, in a wide concentration range of valproic acid, indicated a close response to Nernst in a short time, and in the presence of different metal ions, it acts selectively relative to valproic acid. Reusability, stability, and the dynamic response time are similar to conventional chemical sensors. The application of the designed sensor was investigated by determining valproic acid in the tablets.
Acknowledgments
This study was supported by Islamic Azad University, Tabriz Branch, Iran, and Ilam University of Medical Sciences.
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID:
Elahe Karimi
https://www.orcid.org/0000-0003-0482-1554
HOW TO CITE THIS ARTICLE
Mohammad Taghi Vardini, Naser Abbasi, Arsalan Kaviani, Masoumeh Ahmadi, Elahe Karimi. Graphite Electrode Potentiometric Sensor Modified by Surface Imprinted Silica Gel to Measure Valproic Acid. Chem. Methodol., 2022, 6(5) 398-408
- Eckschlager T., Plch J., Stiborova M., Hrabeta J., J. Mol. Sci., 2017, 18:1414 [Crossref], [Google Scholar], [Publisher]
- Göttlicher M., Minucci S., Zhu P., Krämer O.H., Schimpf A., Giavara S., Sleeman J.P., Lo Coco F., Nervi C., Pelicci P.G., Heinzel T., EMBO J., 2001, 20:6969 [Crossref], [Google Scholar], [Publisher]
- Kang J., Park Y.S., Kim S.H., Kim S.H., Jun M.Y., Korean J. Physiol. Pharmacol., 2011, 15:67. [Crossref], [Google Scholar], [Publisher]
- Amini H., Javan M., Ahmadiani A., Chromatogr. B Anal. Technol. Biomed. Life Sci., 2006, 830:368. [Crossref], [Google Scholar], [Publisher]
- Lin M.C., Kou H.S., Chen C.C., Wu S.M., Hsin w., Chromatogr B Anal. Technol. Biomed. Life Sci., 2004, 810:169 [Crossref], [Google Scholar], [Publisher]
- Gao S., Miao H., Tao X., Jiang B., Xiao Y., Cai F., Yun Y., Li J., Chen W., Chromatogr B Anal. Technol. Biomed. Life Sci., 2011, 879:1939. [Crossref], [Google Scholar], [Publisher]
- Pham T.T.T., See H.H., Morand R., Krahenbuhl S., Hauser P., Chromatogr. B, 2012, 907:74 [Crossref], [Google Scholar], [Publisher]
- Cooreman S., Cuypers E., De Doncker M., Hee P., Uyttenbroeck W., Neels H., Immuno-analyse & Biologie Spécialisée, 2008, 23:240 [Crossref], [Google Scholar], [Publisher]
- Ho T.S., Pedersen-Bjergaard S., Rasmussen K.E., Analyst, 2002, 127:608 [Crossref], [Google Scholar], [Publisher]
- Gupta R., Kumar A., Adv., 2008, 26:533 [Crossref], [Google Scholar], [Publisher]
- Gao R., Su X., He X., Chen L., Zhang Y., Talanta, 2011, 83:757 [Crossref], [Google Scholar], [Publisher]
- Xiao P., Corvini P., Shahgaldian P., Chem., 2010, 2:120 [Crossref], [Google Scholar], [Publisher]
- Tse Sum Bui B., Haupt K., Bioanal. Chem., 2010, 398:2481 [Crossref], [Google Scholar], [Publisher]
- Zakerian R., Bahar S., Iran. Chem. Soc, 2019, 16:741 [Crossref], [Google Scholar], [Publisher]
- Zabihollahpoor A., Rahimnejad M., Najafpour G., Moghadamnia A.A., Electroanal. Chem., 2019, 835:281 [Crossref], [Google Scholar], [Publisher]
- Petit-Domínguez M.D., Quintana C., Vázquez L., del Pozo M., Cuadrado I., Parra-Alfambra A.M., Casero E., Acta, 2018, 185:334 [Crossref], [Google Scholar], [Publisher]
- Bakker E., Telting-Diaz M., Chem., 2002, 74:2781 [Crossref], [Google Scholar], [Publisher]
- Malon A., Vigassy T., Bakker E., Pretsch E., Am. Chem. Soc., 2006, 128:8154 [Crossref], [Google Scholar], [Publisher]
- Saber Tehrani M., Vardini M.T., Abroomand Azar P., Husain S.W., Iran. Chem. Soc., 2010, 7:759 [Crossref], [Google Scholar], [Publisher]
- Jiang N., Chang X., Zheng H., He Q., Hu Z., Chim. Acta, 2006, 577:225 [Crossref], [Google Scholar], [Publisher]
- Ersöz A., Say R., Denizli A., Chim. Acta, 2004, 502:91 [Crossref], [Google Scholar], [Publisher]
- Karimi E., Abbasi N., Abbasi S., Vardini M.T., Methodol., 2021, 5:200 [Crossref], [Google Scholar], [Publisher]
- Widayani, Yanti, Wungu T.D.K., Suprijadi, Procedia Eng., 2017, 170:84 [Crossref], [Google Scholar], [Publisher]
- Prathish K.P., Prasad K., Rao T.P., Suryanarayana M.V., Talanta, 2007, 71:1976 [Crossref], [Google Scholar], [Publisher]
- Arabi M., Ghaedi M., Ostovan A., ACS Sustainable Chem. Eng., 2017, 5:3775 [Crossref], [Google Scholar], [Publisher]
- Luo J., Gao Y., Tan K., Wei W., Liu X., ACS Sustainable Chem. Eng., 2016, 4:3316 [Crossref], [Google Scholar], [Publisher]
- Zhi K., Wang L., Zhang Y., Jiang Y., Zhang L., Yasin A., Materials, 2018, 11:777 [Crossref], [Google Scholar], [Publisher]
- Kalogiouri N.P., Tsalbouris A., Kabir A., Furton K.G., Samanidou V.F., J., 2020, 157:104965 [Crossref], [Google Scholar], [Publisher]
- Zhi K., Wang L., Zhang Y., Jiang Y., Zhang L., Yasin A., Materials, 2018, 11:777 [Crossref], [Google Scholar], [Publisher]
- Ansari S., Ghorbani A., Chem. Health Risks, 2017, 7 [Crossref], [Google Scholar], [Publisher]
- Rao T.P., Kala R., Talanta, 2008, 76:485 [Crossref], [Google Scholar], [Publisher]
- Liu Y., Wei M., Hu Y., Zhu L., Du J., Actuators B Chem., 2018, 255:544 [Crossref], [Google Scholar], [Publisher]
- Ahmad O.S., Bedwell T.S., Esen C., Garcia-Cruz A., Piletsky S.A., Trends Biotechnol., 2019, 37:294 [Crossref], [Google Scholar], [Publisher]
- El-Akaad S., Mohamed M.A., Abdelwahab N.S., Abdelaleem E.A., De Saeger S., Beloglazova N., Rep., 2020, 10:14479 [Crossref], [Google Scholar], [Publisher]
- Khalifa M.E., Abdallah A.B., Bioelectron. X, 2019, 2:100027 [Crossref], [Google Scholar], [Publisher]
- Bakker E., Bühlmann P., Pretsch E., Rev., 1997, 97:3083 [Crossref], [Google Scholar], [Publisher]
- Rosatzin T., Bakker E., Suzuki K., Simon W., Chim. Acta, 1993, 280:197 [Crossref], [Google Scholar], [Publisher]
- Soleymanpour A., Rad N., Niknam K., Actuators B Chem., 2006, 114:740-746 [Crossref], [Google Scholar], [Publisher]
- Sadeghi S., Fathi F., Esmaeili A.A., Naeimi H., Actuators B Chem., 2006, 114:928 [Crossref], [Google Scholar], [Publisher]
- Mosallanejad B., Methodol., 2019, 3:261 [Crossref], [Google Scholar], [Publisher]
- Mazloum M., Niassary M.S., Amini M.K., Actuators B Chem., 2002, 82:259 [Crossref], [Google Scholar], [Publisher]
- Karami H, Mousavi MF, Shamsipur M, Talanta, 2003, 60:775 [Crossref], [Google Scholar], [Publisher]
- Somer G., Kalaycı S., Ekmekci G., Actuators B Chem., 2001, 81:122 [Crossref], [Google Scholar], [Publisher]
- Khan A.A., Inamuddin, Actuators B Chem., 2006, 120:10 [Crossref], [Google Scholar], [Publisher]
- Shamsipur M., Mizani F., Saboury A.A., Sharghi H., Khalifeh R., Electroanalysis, 2007, 19:587 [Crossref], [Google Scholar], [Publisher]
- Bakker E., Pretsch E., Bühlmann P., Chem., 2000, 72:1127 [Crossref], [Google Scholar], [Publisher]
- Umezawa Y., Bühlmann P., Umezawa K., Tohda K., Amemiya S., Pure Appl. , 2000, 72:1851 [Crossref], [Google Scholar], [Publisher]
- Khameneh Asl S., namdar m., Methodol., 2019, 3:183 [Crossref], [Google Scholar], [Publisher]


