Document Type : Original Article
Authors
1 Department of Chemistry, Graduate University of Advanced Technology, Kerman, Iran
2 Environment Department, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran
Abstract
In this work, three dimensional NiO nanowrinkles (3D NiO-NWs) were prepared and used as electrode materials to modify the surface of a glassy carbon electrode (3D NiO-NWs/GCE). Then, differential pulse voltammetry (DPV), cyclic voltammetry (CV) and chronoamperometry (CHA) were employed to determine the electrochemical response of theophylline on as-fabricated sensor. The electrochemical theophylline oxidation was elevated on the modified electrode. The peak current on the modified electrode in phosphate buffer solution (PBS, 0.1 M, pH=7.0) showed a linear elevation with an increase in the theophylline concentration (0.1-900.0 µM), with a narrow detection limit of 0.03±0.001 µM.
Graphical Abstract
Keywords
- Theophylline
- Three Dimensional NiO Nanowrinkles
- Glassy carbon electrode
- Differential pulse voltammetry
Main Subjects
Introduction
1,3-Dimethylxanthine, or theophylline, is a member of xanthine family, with a great influence on respiration. Theophylline is extensively administered to manage different asthmatic problems as a bronchodilator agent. Theophylline acts as a successful respiratory stimulant to treat neonatal apnea, pediatric acute asthma, signs of acute and chronic asthma, bronchospasm [1], chronic obstructive pulmonary disease, and emphysema in adults. The acceptable limit of theophylline has been set at 5-20 μg/mL in adults for effectual treatment [2]; exceeding this range may be associated with adverse events, such as tachycardia, vomiting, central nervous system irritation and seizures. Theophylline, similar to other derivatives of methylated xanthine, is not only a competitive nonselective phosphodiesterase inhibitor [3], but also is a nonselective adenosine receptor antagonist [4]. Accordingly, there is a need for development of a facile analytical protocol to sensitively detect the theophylline. The existing techniques for the quantification of theophylline include spectrophotometry [5], high-performance liquid chromatography [6], chemiluminescence [7], surface-enhanced raman scattering [8], and gas chromatography–mass spectrometry [9]. All these methods, in addition to many advantages, suffer from some disadvantages such as complexity and cost- and time-consuming requisites. Among these, the electrochemical systems have aroused widespread attentions owing to rapidity, excellent sensitivity, time saving, negligible energy consumption, adorable reliability, affordability and ease of use [10-20].
The electrochemical sensors can have potent controllable properties through chemically surface modification of inert electrode substrates [21-28]. In operation, redox active sites move electrons between the analyte solution and the electrode substrates, mostly with a remarkable decrease in overpotential of activation. Moreover, chemically modified electrodes are less prone to oxide formation and surface fouling when comparing with inert electrode substrates.
Nanotechnology has enormous potential for providing innovative solutions to a wide range of applications [29-33]. Development of nanoscience and nanotechnology has allowed trials to apply different nanomaterials for the fabrication of chemically modified electrodes. In recent years, metal oxide nanoparticles (NPs), metallic (like silver and gold) NPs), and carbon nano-structures, like graphene and carbon nanotubes, alone or in combination have been utilized for electrode surface modification [34-40].
A metal oxide possessing great electrocatalytic response, as a p-type NiO semiconductor, has
a band-gap energy between 3.6 and 4 eV [41]. They have special merits, such as impressive electro-activity, exceptional electrochromic efficiency, admirable electro-conductivity, huge surface area, cost-effective fabrication process, and a broad range of modulation. Accordingly, NiO has been a successful electron mediator in the construction of various electrochemical sensing systems [42-44].
In this paper, the analytical application of a GCE modified with 3D NiO-NWs is proposed for determining theophylline. The modified electrode could remarkably enhance the electrochemical responses of theophylline and improve the sensitivity of theophylline detection.
Materials and Methods
Instruments and chemicals
PGSTAT-302N Autolab potentiostat/galvanostat (Eco Chemie, Netherlands) was a device to perform all electrochemical tests whose data were analyzed by General Purpose Electrochemical System (GPES) software. The phosphate buffer solutions within the PH value of 2.0 to 9.0 were prepared by the orthophosphoric acid and relevant salts (KH2PO4, K2HPO4, K3PO4). The pH values were measured by a digital pH meter (Metrohm type 713) equipped with a combined glass electrode in all solutions. The analytical grade of chemicals such as theophylline and other reagents were from Sigma-Aldrich and used as received.
Preparing the electrode
The 3D NiO-NWs were used for the modification of a GCE to prepare 3D NiO-NWs/GCE. For this purpose, 3D NiO-NWs suspension (1 mg/mL) in deionized water was prepared and 4 μL of the 3D NiO-NWs suspension drop was cast onto the GCE surface and allowed to dry completely in ambient conditions.
Results and Discussion
Electrochemical behaviour of theophylline at the various surface of electrodes
The electrochemical behavior of theophylline is proportional to the aqueous solution pH (Scheme 1). Hence, it appears necessary to optimize the solution pH to reach the best electrocatalytic oxidation of theophylline. Therefore, DPV was used to explore the electrochemical response of theophylline on the surface of 3D NiO-NWs/GCE in 0.1 M PBS at various pH values (2-9). According to the results, the highest peak current was related to the pH value of 7 (the optimum one) for theophylline oxidation on the 3D NiO-NWs/GCE surface.
The electrochemical performance of 3D NiO-NWs/GCE in comparison with bare GCE was studied by the CV technique in exposure to 200.0 μM of theophylline at 50 mV/s in PBS (0.1 M). The CVs of all as-fabricated electrodes in this study can be observed in Figure 1. The 3D NiO-NWs/GCE voltammetric behaviour (curve b) exhibited the relatively strongest and most characteristic anodic peak at 850 mV, sequentially. The bare GCE voltammetric behaviour (curve b) exhibited the relatively weak oxidation peak with a less intense at 1000 mV, sequentially. Consequently, the 3D NiO-NWs/GCE has obviously better electrocatalytic behaviour than the bare GCE towards the theophylline with the relatively strong current response.
The effects of the scan rate
The CV method was used to explore the influence of scan rate on the oxidation process of theophylline (100.0 μM) in 0.1 M PBS, as shown in Figure 2. An elevation in the scan rate (10-600 mV/s) enhanced the oxidation peak current of theophylline, in addition to gradually shifting the anodic potentials to more positive values. Figure 2 (inset) emphasizes the proportionality of the anodic response current linearly to the scan rate square root, which shows controlled-diffusion processes for the electrocatalytic oxidation of theophylline on 3D NiO-NWs/GCE.

Figure 1: CVs captured for 3D NiO-NWs/GCE (a) and bare GCE (b) in PBS (0.1 M, pH = 7.0) in exposure to 200.0 μM of theophylline at 50-mV/s the scan rate

Figure 2: CVs captured for 3D NiO-NWs/GCE in PBS (0.1 M, pH = 7.0) in exposure to 100.0 μM of theophylline at various scan rates; (1): 10, (2): 30, (3): 70, (4): 100, (5): 200, (6): 300, (7): 400, (8): 500, and (9): 600 mV/s, sequentially; Inset: changes in anodic peak currents against ν1/2
Chronoamperometric measurements
Chronoamperometry was considered for the investigation of theophylline electro-oxidation at the potential of 900 mV and various analyte concentrations in PBS (pH = 7.0), as seen in Figure 3. The current response of electroactive theophylline was described under a diffusion-limited electrocatalytic process using Cottrell’s equation:
I = nFAD1/2Cbπ-1/2t-1/2
In this equation, n stands for the number of electron transfer exchanged per reactant molecule, F for the Faraday constant, Cb for sunset yellow concentration (mol/cm3), and D for diffusion coefficient (cm2/s). The plot of I versus t−1/2 provided various linear curves for different theophylline contents of 0.1-1.0 mM (Figure 3, inset A). The slope of each straight line against the theophylline content eventually made it possible to calculate the overall slope of the best-fit line (Figure 3, inset B). At last, the overall slope in the Cottrell’s equation was utilized to estimate the mean D value, which was 1.6±0.02 ×10−6 cm2/s.

Figure 3: Chronoamperograms for 3D NiO-NWs/GCE in PBS (0.1 M, pH = 7.0) for different theophylline contents; (1): 0.1, (2): 0.4, (3): 0.7, and (4): 1.0 mM of theophylline; Insets: (a) Plots of I against t−1/2 from chronoamperograms 1 to 4. (b) Plot of straight lines slope against theophylline content
Calibration plot and limit of detection
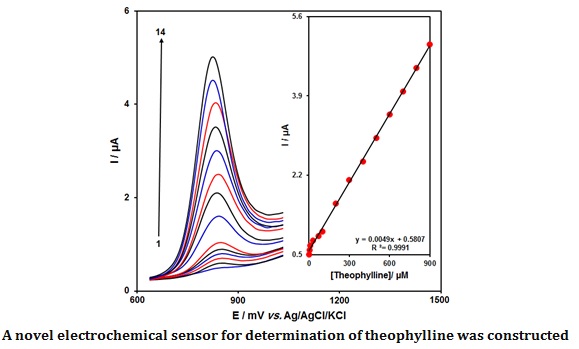
The theophylline content was measured by the DPV technique. The DPVs captured for 3D NiO-NWs/GCE at various theophylline contents in PBS (0.1 M) are shown in Figure 4. There was a stepwise enhancement in the theophylline oxidation current by gradually increasing the theophylline contents, meaning the applicability of 3D NiO-NWs/GCE for electrochemically sensing the theophylline. Figure 4 (inset) represents the alterations in the oxidation signal on the 3D NiO-NWs/GCE as a function of various theophylline contents (0.1-900.0 µM), having a low limit of detection of 0.03 μM.
Also, the detection limit, Cm, of theophylline was obtained using the following equation:
Cm=3Sb/m
In the above equation, m is the slope of the calibration plot (0.0049 μA/ μM) and Sb is the standard deviation of the blank response, which is obtained from 20replicate measurements of the blank solution. The detection limit is 0.03±0.001 µM. The comparison of the results for the detection of theophylline with different modified electrodes in the literatures is listed in Table 1.

Figure 4: DPVs captured for 3D NiO-NWs/GCE in PBS (0.1 M, pH = 7.0) in exposure to various theophylline contents; (1): 0.1, (2): 5.0, (3): 10.0, (4): 30.0, (5): 70.0, (6): 100.0, (7): 200.0, (8): 300.0, (9): 400.0, (10): 500.0, (11): 600.0, (12): 700.0, (13): 800.0, and (14): 900.0 µM of theophylline; Inset: plot of peak current as a function of various theophylline contents (0.1-900.0 µM)
Table 1: Comparison of the determination of theophylline between 3D NiO-NWs/GCE and some previous works reported in the literature
|
Sensor |
Analytical methods |
Linear range |
LOD |
Ref |
|
A Nafion/multi-wall carbon nanotubes (MWNTs) composite film-modified glassy carbon electrode |
DPV |
8⋅0 × 10–8–6⋅0 × 10–5 M |
2⋅0 × 10–8 M |
|
|
Urchin-like CdSe microparticles modified glassy carbon electrode |
DPV |
1.0-700.0 µM |
0.4 µM |
|
|
boron-doped diamond electrode |
SWV |
2.0-380.0 µM |
1.45 µM |
|
|
DPV |
0.91 µM |
|||
|
manganese oxide nanoparticles/multiwalled carbon nanotube nanocomposite modified glassy carbon electrode |
DPV |
0.1-20.0 µM |
0.01 µM |
|
|
MnO2nanosheets/ionic liquid-functionalized grapheme/ glassy carbon electrode |
DPV |
1.0-220.0 µM |
0.1 µM |
|
|
Tungsten trioxide nanoparticles_multiwall carbon nanotubes/ glassy carbon electrode |
DPV |
0.025-2.6 µM |
0.008 µM |
|
|
poly-sulfosalicylic acid film decorated pure carbon fiber/ glassy carbon electrode |
DPV |
0.6-137.0 µM |
0.2 µM |
|
|
3D NiO-NWs/GCE |
DPV |
0.1-900.0 µM |
0.03 µM |
This Work |
Conclusion
In summary, a GCE was modified using 3D NiO-NWs to provide a sensitive electrochemical sensor in the theophylline sensing. Based on the findings, the 3D NiO-NWs/GCE possessed a potent electrocatalytic performance toward theophylline. In the optimized circumstances, the linear range was broad from 0.1 to 900.0 μM for theophylline and the limit of detection was 0.03±0.001 μM.
Acknowledgments
The authors wish to thank Graduate University of advanced technology, Kerman, Iran for finantioal support of this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting, and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID
Hadi Beitollahi
https://orcid.org/0000-0002-0669-5216
HOW TO CITE THIS ARTICLE
Effat Sharifi Pour, Maryam Ebrahimi, Hadi Beitollahi. Electrochemical Sensing of Theophylline using Modified Glassy Carbon Electrode, Chem. Methodol., 2022, 6(7) 560-568