Document Type : Original Article
Authors
1 Department of Chemistry, College of Education of Pure Science, Ibn – Al Haitham, University of Baghdad, Iraq
2 Head of Department of Medical Laboratory Techniques, Ashur University College, Baghdad, Iraq
Abstract
Bismuth oxide nanoparticle Bi2O3NPs has a wide range of applications and less adverse effects than conventional radio sensitizers. In this work, Bi2O3NPs (D1, D2) were successfully synthesized by using the biosynthesis method with varying bismuth salts, bismuth sulfate Bi2(SO4)3 (D1) or bismuth nitrate. Penta hydrate Bi(NO3)3.5H2O (D2) with NaOH with beta-vulgaris extract. The Bi2O3NPs properties were characterized by different spectroscopic methods to determine Bi2O3NPs structure, nature of bonds, size of nanoparticle, element phase, presence, crystallinity and morphology. The existence of the Bi2O3 band was verified by the FT-IR. The Bi2O3 NPs revealed an absorption peak in the UV-visible spectrum, with energy gap Eg = 3.80eV. The X-ray pattern (D1) matching that of card of COD File-No-96-152-6459 indicating the presence of homogeneous Bi2O3NPs, Scaning Electron Microscopy (SEM) displayed shaped monoclinic phase with average diameter 30.28 nm. The size, structure and composition of synthetic Bi2O3 Nps were determined using the (EDX) pattern. The XRD pattern (D2) corresponds to JCPDS File No. 27-50. The SEM of D2 showed crystalline rhomobohedrral phase with average diameter 34.89 nm. The EDX for both (D1, D2) samples reveals an aggregation of thin sheet cluster. The antibacterial activity of Bi2O3NPs from (D1, D2) was tested against (G-) Escherichia coli and (G+) staphylococcus aureus. All of these clinical pathogens were examined for antifungal activity against Candida albicans fungus, and the results were compared with the standard medication. The adsorption experiment was successfully conducted on the following metal ions (M+2 = Co, Ni and Cu), where the results proved removal simultaneously from water using Bi2O3NPs (D1, D2) based on the affinity of three metal ions and Bi2O3 NPs surface shape. The removal efficiencies of mixed (M+2 = Co, Ni and Cu) ions for D1 were 89.68%, 85.56% and 94.5%. The removal efficiencies for D2 were 93.3%, 87.7% and 88.54%, respectively.
Graphical Abstract
Keywords
Main Subjects
Introduction
Metal oxide NPs (MO – NPs) have proved to be particularly effective in inhibiting bacterial growth [1-3] and antibacterial resistance, treatment to remove multiple heavy metal ions from waste water by using Fe3O4NPs, gas sensor, and catalyst [4-6]. Manufacture oxide nanoparticles with specified sizes and shapes, green synthesis is a challenge [7, 8]. Because of their ease of use, abundant biodiversity and eco- friendly processes, biosynthesis technologies offer a distinct advantage over other traditional synthesis approaches. [9, 10]. Mentha Puegium (pennyroyal) leaf extract was used to make silver nanoparticles for antibacterial use [11, 12]. Bi2O3 NPs was synthesized via green synthesis using Mentha Pulegium aqueous extract and it showed antibacterial activities [7]. Researchers have synthesized Bi2O3 NPs by using Jotopha multifidi leaf extract. The Bi2O3 NPs that were generated contain a monoclinic structure, a 3.34 eV optical band gap, agglomeration morphology and a particle size of 17.26 nm [13]. At room temperature, the researchers biosynthesized Bi2O3 NPs in a size range of 5-8 nm by fungus – fusarium oxysporum with Bi(NO3)3.5H2O [14]. In the present study, we have developed a facile green synthesis method for preparation of Bi2O3 NPs using beta vulgaris extract. The objective of this research was to achieve the goals of green synthesis for potential application and antibacterial activity used as adsorbent to metal ions.
Materials and Methods
Sample collection
Beta vulgaris was taken from a nearby source and marked. Bismuth nitrate. penta hydrate Bi (NO3)3.5H2O and Bismuth sulfate, Bi2(SO4)3 were purchased from Germany/ Merck, NaOH form Alpha India/ Alpha Chemica, Ethanol from Spain/ RBL, also Cobalt sulfate CoSO4, Copper sulfate. Penta hydrate CuSO4. 5H2O and Nickel sulfate. Hepta hydrate NiSO4.7H2O. All chemicals employed without additional purification.
Various spectroscopic and microscopic approaches were used to synthesize and identify Bi2O3NPs as follows: A Sensitive Electronic Balance type RADWAG, model / As 220 /C / 1, Magnetic stirrer, Centrifuge type PLC, (4000 – 4500 rpm) [(66–90 w) power: 110 L 60 Hz, 230 L 50 Hz], Electric oven type (FAITHFUL) model – WHL. 25 AB, Shaking water bath type (SCL FINETEDI), Tape measure of PH, (UV-vis) kind, (160/ Uv) Shimadzu spectroscopy, FT-IR (8500S) type Shimadzu at university of Baghdad / Collage of science and (XRD) X-ray diffraction type (Phillips/ Holland) were examined in the laboratories of the (center of examinations) at Baghdad. SEM with Field Emission (FE) type (Hv 300/ Ziess sigma -Germany) and X-ray energy dispersion device (EDX) were applied. Transmission electron microscope (TEM) (kind/ EM10C-100Kv Germany) was put to test at Kashan University in Iran. The antimicrobial activity of the synthetic Bi2O3 NPs was tested against two reference bacterial strains, (G+) Staphylococcus aureus, and (G-), Escherichia coli and, as well as Candida albicans fungal, using the disc diffusion method in a nutrient medium (jellose medium) type Muller Hinton agar, and the same method was used for antifungal activity using the nutrient medium (agar) potato dextrose PDA.
Bi2O3NPs preparation by (Beta vulgaris extract) with Bi2(SO4)3 and Bi(NO3)3. 5H2O (D1 and D2)
Fresh Beta vulgaris must be first prepared. Beta vulgaris was rinsed with tap water to eliminate any contaminants and dried at 37 °C for one day. About 20 g of beta vulgaris was poured in a 500 mL beaker with 200 mL deionized water, mixed thoroughly and heated at 90 °C for 30 minutes. The extracted solution's hue changed from maroon to red-purple when it was allowed to cool to ambient temperature. Whatman filter paper No. 1 was used to filter the mixture. The resultant solution was centrifuged for 15 minutes at 4000 rpm in a 1.5 mL tube. Beta vulgaris extract stock solution was obtained. (7.06 g) of Bi2(SO4)3 or Bi(NO3)3.5H2O was dissolved in 30 mL deionized water, then 100 mL Beta-vulgaris extract was added as dispersant, with stirring and heating at a temperature 90 Celsius, then a solution of (1 M) NaOH was gradually added to it drop by drop by dissolving 2 g of NaOH in 500 mL of deionized distilled water until the pH value turned 12. The mixture was left for 48 hours in order to precipitate, forming a pale green precipitate. Next, separating was done by using a centrifuge, washing the precipitate with hot distilled water and hot EtOH, and getting it dried at a temperature of 300 Celsius by using an electric oven for 10 hours, leaving a pale brown powder of D1 and yellow powder of D2.
Results and Discussion
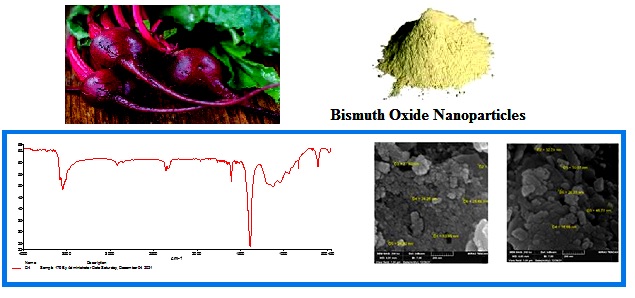
The two samples (D1 and D2) were analyzed using FT-IR spectrum. Figure 1 shows absorption bands at 600 cm-1, both of which were due to the stretching mode of Bi-O while the peak of stretching vibration of O-H appeared at 3429 cm-1. Peaks at 2900 cm-1, 2800 cm-1 and 1113 cm-1, 1046 cm-1 are attributed to vibrations of CH2 aliphatic and C-O bond respectively of beta vulgaris precursor [7, 15].
Spectra for both D1, D2 confirmed the presence of Bi2O3NPs. As shown in Figure 2 at 326 nm, an absorption peak was seen, attributed to Bi2O3NPs. The energy gap was calculated by using equation Eg = 1239.83 / λ. Eg is the bulk band expressed in eV. λ = lambada is peak absorbsotion, Eg = 1239.83 / 326 = 3.80 eV [7].

Figure 1: FT – IR spectrum of Bi2O3NP for (D1, D2 samples)

Figure 2: UV-Vis spectrum of Bi2O3 Nps
Pattern of Bi2O3 Np was synthesized from Bi2(SO4)3 (D1) of beta vulgaris. Figure 3 gives peaks indicating that the Bi2O3 Np are amorphous. The peaks are associated with the monoclinic crystal phase of Bi2O3 (COD: No-96-152-6459) at the 2θ value of 28.56, 34.35, 42.52, 46.50 and 58.58 [13].

Figure 3: XRD of Bi2O3 Nps for (D1 sample)
The Bi2O3 NPs was synthesized from Bi(NO3)3.5H2O (D2) of beta vulgaris. Figure 4 showed rhombohedral phase of Bi2O3 suitable with JCPDS file - 27- 50 (Bi2O3) at the 2θ value of 27.66, 31.39, 32.501, 45.89, 55.34, and 74.45. The Debye Scherres equation was used to compute the average crystallite size of both samples, and the average crystal size of D1 and D2 was found to be 30.284 nm and 34.896 nm, respectively [16].
The morphology studies of both Bi2O3 samples (D1 and D2) are shown in Figure 5. The image shows that spherical particles tend to aggregate with average size of the particles by 30.28 nm and 34.89 nm [17].
The elemental and compositional properties of both sample of Bi2O3NPs were studied by EDX. The EDX spectra of Bi2O3NPs were synthesized by beta vulgaris extracts. Figure 6 depicts the discovery of strong bismuth (Bi) peaks at 2.5 and a medium peak of oxygen (O) at 0.5. [13].

Figure 4: XRD of Bi2O3 Nps for (D2 sample)

Figure 5: Image of SEM Characterized for D1 and D2

Figure 6: EDX of Bi2O3 Nps for (D1, D2)
The morphology of Bi2O3Nps was studied using a transmission electron microscope (Figure 7). TEM image reveals an aggregation of thin sheet clusters [18].
Adsorption activity of Bi2O3NPs
The use of nanomaterials to remove various metal ions present in water simultaneously opens up a new path that is free of secondary contamination and is very affordable. Bi2O3 NPs has a large specific surf ace area, surface area per unit mass, porous material and the structure and surface shape of Bi2O3 NPs, as seen in Figures 6 and 7 (SEM TEM), making it a strong adsorption candidate. At room temperature and pH = 6, three metal ions, namely Co+2, Ni+2, and Cu+2, were effectively removed from water concurrently using Bi2O3NPs. The removal of these metal ions from water is shown in this research to have a quick adsorption of Co+2, Ni+2 and Cu+2, with more than 90% adsorption in less than a minute for Ni+2. As seen in Figure 8, the rate of metal ions uptake steadily declined and did not appear to grow any more throughout this time. These findings may be explained by the fact that there were more active sites available on the Bi2O3 NPs, adsorbent, at the start of the adsorption process, and as time passed, these sites gradually became saturated [19, 20]. For the D1 sample, the removal efficiencies of Co(II), Ni(II) and Cu(II) ions were 89.68%, 85.56%, and 94.56%, respectively and for sample D2, the removal efficiencies of Co(II), Ni(II) and Cu(II) ions were 93.3%, 87.7 % and 88.54 %, respectively.

Figure 7: Image of TEM characterized for D1 and D2

Figure 8: The removal efficiencies of Co(II), Ni(II), and Cu(II) ions for D1and D2 sample
Antibacterial activity of Bi2O3 NPs
Bismuth is found as [Bi2O3, Bi2 (CO3)3, Bi2S3] in medicine. Bismuth has been employed as an antidiarrheal to treat vomiting, stomach, and pain nauseas. Bi2O3NPs porous Nano spheres demonstrated inactivation of (G–) and (G+) bacteria [21, 22]. To perform a qualitative antimicrobial screening, agar well diffusion and minimum inhibitory concentration experiments are carried out. The zones of inhibition at diluted concentration of HCl (0.02g / mL) for D1 are obtained as E. Coli and S. aures is (31 and 29 mm) for D1 is (30 and 30 mm), candida albicans (43 and 45 mm) of D1 and D2 for Bi2O3NPs preparation. After this compressive analysis, we concluded that both Staphylococcus aureus and E. Coli have the highest inhibitory values with sample D1 and D2 and also a very high inhibition with Candida albicans fungal for the same samples as shown in Figure 9 [23, 24].

Figure 9: Types of bacteria and fungi tested and inhibition diameters in units (mm) of prepared Bi2O3NPs for D1 and D2 samples
Conclusion
Bi2O3NPs were successfully synthesized by a simple cost-effective biosynthesis method of Bi2O3 monoclinic phase with diameter of 30. 284 nm produced by using Bi2(SO4)3. Bi2O3 rhombohedral phase with diameter (34.896) nm was obtained by using Bi(NO3)3.5H2O. The morphology of Bi2O3NPs was as aggregation of thin sheet cluster. They have antimicrobial activity inhibiting the growth of S. aures, E. Coli and a very high inhibition with Candida albicans fungal. Bi2O3NPs were successfully removed simultineously with three metal ions (M+2 = Co, Ni and Cu) from water contaminated with them.
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
HOW TO CITE THIS ARTICLE
Aqeel O. Flayyih, Waleed K. Mahdi, Yousif. I. M. Abu Zaid, Falih H. Musa. Biosynthesis, Characterization, and Applications of Bismuth Oxide Nanoparticles Using Aqueous Extract of Beta Vulgaris. Chem. Methodol., 2022, 6(8) 620-628