Document Type : Original Article
Authors
Department of Chemistry, Ardabil Branch, Islamic Azad University, Ardabil, Iran
Abstract
In this study, the capability to adsorb and detect of pure and doped Al12N12 nanoclusters were utilized to detect sarin nerve agents using the density functional theory (DFT) calculation. After adsorption of sarin on the Al12N12, SiAl11N12, and GaAl11N12 nanoclusters, the adsorption energies (Ead) were calculated at -38.36, -25.95, and -27.94 kcal mol-1, respectively. The electrical properties calculations indicated electrical conductivity changes after adsorption of sarin are -3.04%, -24.64%, and -1.16% for Al12N12, SiAl11N12, and GaAl11N12, respectively. Thus, it is clear that the SiAl11N12 nanocluster demonstrated a considerable change in the electrical conductivity, and these changes could be considered as the signal for the sarin detection. Moreover, SiAl11N12 indicated an ideal recovery time for sarin desorption from nanoclusters. Thus, it can be concluded that the SiAl11N12 nanocluster is an appropriate candidate for the sarin detection.
Graphical Abstract
Keywords
Main Subjects
Introduction
Of the chemical warfare agents, the nerve agent is the most hazardous [1]. They influence fast on human health and lead to death [2, 3]. Sarin, as a dangerous nerve agent, is a potent acetylcholinesterase inhibitor [4]. Due to be odorless, tasteless, and colorless detection of sarin is very hard [5]. Thus, designing a reliable and facile technique for the sarin detection is highly essential.
For sarin detection, several techniques, such as chromatography [6], photo luminescent [7], mass spectroscopy [8], and fluorescent sensors [9] have been utilized. Nevertheless, these techniques need more energy and time and are expensive. Compared with these techniques, chemical sensors based on the nanostructures have been used widely due to their short response time, high selectivity, affordable, and comfortable applications [10-15]. Thus, nanoclusters have recently been considered as an alternative approach to the chemical sensors [16-21]. Among different kinds of nanoclusters, aluminum nitride (AlN) nanostructures were extensively used due to the wide bandgap, economic efficiency, and thermal stability [22-24].
The AlN nanostructures could be modified by loading noble metals and doping atomic impurities [25-29]. For example, Nayini et al. investigated the sensitivity and reactivity of pure and Si-, Ga-, and Ge-doped AlN nanoclusters with amphetamine drugs using density functional theory (DFT) calculations [26]. They indicated that Si-doped AlN is a potential nanocluster for the amphetamine detection. Thus, in this study, we investigated the adsorption and detection properties of sarin on the Al12N12, Si-doped AlN (SiAl11N12), and Ga-doped AlN (GaAl11N12) nanoclusters using the DFT calculations.
Computational method
The sarin interaction with the Al12N12, SiAl11N12, and GaAl11N12 nanoclusters surfaces was calculated using the DFT calculations. The GAMESS program [30] and the M06 method with an and 6-311G (d, P) basis set were utilized for all investigations. Reports indicated that the M06 method is very reliable due to the excellent agreement with the experimental results. The previous reports have indicated that the M06 method is better than the other methods, such as B3LYP, MP2, B9W91, QCISD, and QCISD (T) [31, 32]. 6-311G (d, p) basis set has been known suitable for nanostructure systems [33, 34]. The adsorption energies (Ead) of sarin onto the Al12N12, SiAl11N12, and GaAl11N12 nanoclusters are as follow:
Ead=E(sarin/Al12N12)-E(Al12N12)-E(sarin) (1)
Ead=E(sarin/SiAl11N12)-E(SiAl11N12)-E(sarin) (2)
Ead=E(sarin/GaAl11N12)-E(GaAl11N12)-E(sarin) (3)
Where, E(sarin/Al12N12), E(sarin/SiAl11N12), and E(sarin/GaAl11N12) are the total energies of the sarin interacted Al12N12, SiAl11N12, and GaAl11N12 nanoclusters, respectively. E(sarin), E(Al12N12), E(SiAl11N12), and E(GaAl11N12) are the total energy of pristine sarin, Al12N12, SiAl11N12, and GaAl11N12, respectively.
Results and Discussion
Adsorption of sarin on Al12N12
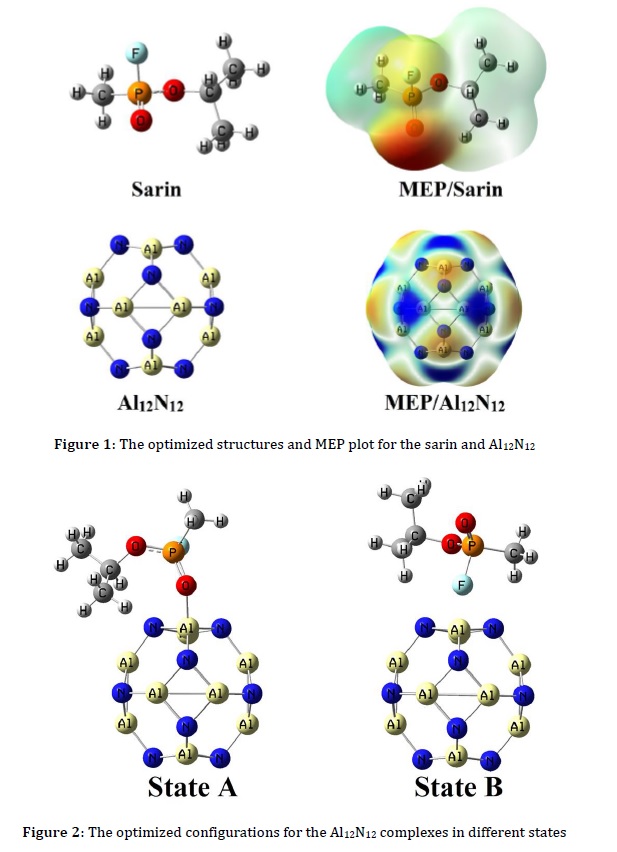
The molecular electrostatic potential (MEP) plot of sarin in Figure 1 indicated fluorine (F) and carbonyl oxygen atoms have a more negative charge (red color) and make it an electronegative site for interaction with the electrophilic region.
Figure 1 demonstrated the most stable structure of Al12N12 with six-membered (6-R) and four-membered (4-R) rings. The bond lengths of the 6R-4R and 6R-6R mutual bonds of Al-N atoms were calculated at 1.84 and 1.78 Å, which is consistent with previous reports [35]. The MEP plot of Al12N12 was shown that the Al and N atoms are electrophilic (blue color) and nucleophile (red color) regions, respectively. To investigate the adsorption properties of sarin with nanoclusters, various possible adsorption sites of sarin (F and carbonyl oxygen atoms) were considered for the interaction with Al12N12 nanoclusters. After optimization, the adsorption energy and bound distance between the nanocluster and sarin from its carbonyl oxygen atom (state A) were calculated at -38.36 kcal.mol-1 and 1.89 Å, which indicated an appropriate interaction. The sarin adsorption from its F atom was calculated in state B (Figure 2). Adsorption energies in state B were investigated at -20.62 kcal.mol-1, and equilibrium distances were 2.97 Å (Table 1). Therefore, these parameters demonstrated the adsorption of sarin from its carbonyl oxygen atom (state A) is stronger since the equilibrium distance between sarin and the Al12N12 nanocluster was shorter and the adsorption energy was more negative. The AlN nanocluster dipole moment (DM) value increased after the adsorption of sarin from 0.00 Debye in pure Al12N12 to 8.79 and 3.70 Debye, representing polar bonds between nanocluster and sarin.
Adsorption of sarin on the SiAl11N12 and GaAl11N12 nanoclusters
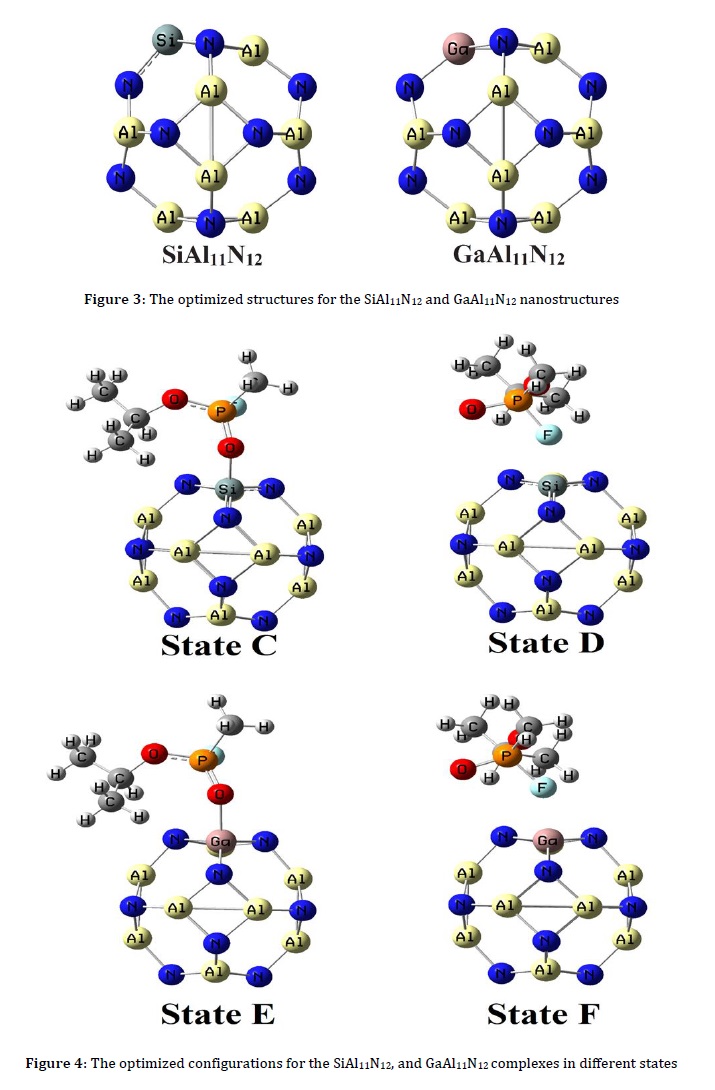
To investigate the doping effect on the adsorption process, Si and Ga atoms were doped into the Al12N12 nanocluster (Figure 3). The previous reports indicated Si and Ga atoms could be doped in the AlN nanoclusters [26]. The Si-O and Ga-O bond lengths in the 6R-4R and 6R-6R mutual bonds indicated a bond length of 1.84 and 1.78 Å, which is consistent with reports [36, 37].
After the sarin adsorption on the SiAl11N12, adsorption energies (equilibrium distances) were calculated at -25.95 (1.82 Å) and -3.71 kcal.mol-1 (2.24 Å) in states C and D (Figure 4). The adsorption energies for GaAl11N12 in states E and F were investigated at -27.94 and -12.47 kcal.mol-1, respectively, and the equilibrium distances were calculated at about 2.02 and 2.25 Å. These values indicated similar to the Al12N12 adsorption of sarin from its carbonyl oxygen atom is stronger than the other states, and this orientation was the most stable state. The NBO charge transfers calculation indicated charge transfer from sarin to the nanoclusters. The DM values in Table 1 indicated that after the sarin adsorption on the SiAl11N12 and GaAl11N12, the values increase.
Electronic characteristics before and after interactions of nanoclusters
In Table 1, the electronic characteristics (HOMO, LUMO, and energy gap (Eg)) of pure and complexes of nanoclusters were provided. The HOMO and LUMO energies of Al12N12 were calculated at -6.80 and -2.23 eV. Thus, the Eg energy (LUMO-HOMO) will equal 4.57 eV. Hoseininezhad-Namin et al. calculated the HOMO, LUMO, and Eg values of Al12N12 at -8.54, -0.63, and 7.91 eV, respectively, at the wB97XD method and 6-311G (d, p) basis set [38]. After doping Si and Ga atoms, the HOMO and LUMO values were changed. Furthermore, Eg values after the doping process reduced -45.51% and -2.45% in the SiAl11N12 and GaAl11N12 nanoclusters, respectively. Based on the previous experimental studies, the change in the sensor's signals is due to the electrical conductivity (σ) alteration after adsorption.
A shift in the electronic properties of AlN nanoclusters could change the electrical conductivity of these nanoclusters, as shown by the following equation with Eg:
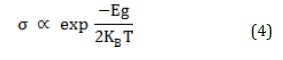
Where, T and KB are the temperature and Boltzmann constant, respectively. According to Equation (4), when Eg decreases after adsorption of sarin, the electrical conductivity increases. This ratio of changes could be investigated by equation (5):
![]()
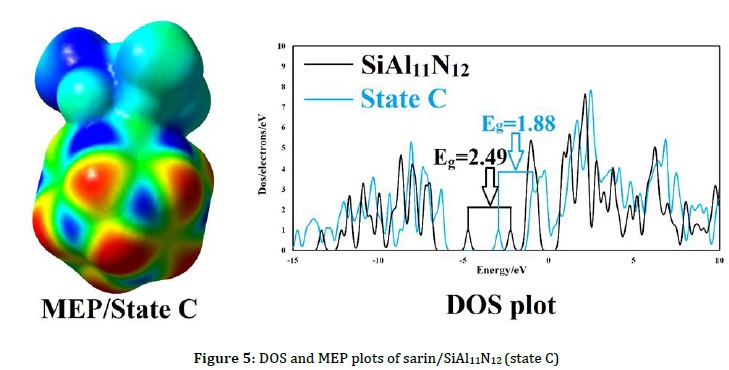
Where, σ0 and σ are the electrical conductivity of pure nanoclusters and complexes, respectively. The %Δσ shows the signal strength, corroborating the sarin's existence. As indicated in Table 1, the %Δσ after the interaction of nanoclusters with sarin is -3.04%, -24.64%, and -1.16% for Al12N12, SiAl11N12, and GaAl11N12, respectively, in the most stable states. Thus, it is clear that SiAl11N12 has higher performance for sarin detection than Al12N12 and GaAl11N12. DOS and MEP plots of the SiAl11N12 complex are indicated in Figure 5. As displayed in Figure 5, after the sarin interaction with SiAl11N12, the DOS plots were changed in HOMO and LUMO energy levels and changed to the lower energies. Thus, the HOMO and LUMO energies stabilized after adsorption and altered the Eg region. MEP plot of the SiAl11N12 complex in Figure 5 demonstrated the significant change in the electrostatic potential after the interaction. This Figure indicated that sarin is more positive (blue and green colors) after the adsorption processes. Therefore, these results demonstrated charge transfers from sarin to the SiAl11N12 nanoclusters, which confirm the charge transfer values.
Recovery time
The interaction strength is essential in developing sensors because a strong adsorption leads to the long-term desorption, and thereby increasing the recovery time. In experimental studies, sensor recovery time can be calculated through methods including heating or exposure to the UV light [39]. Thus, the recovery time could be investigated from the following equation:
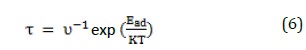
Where, T is the temperature, k is the Boltzmann's constant, and ν indicates UV frequency. Therefore, the recovery time for Al12N12, SiAl11N12, and GaAl11N12 complexes will be about 12.37 × 109, 9.93, and 284.68 s at the UV frequency of 1018 s-1 and 298 K, respectively. These values showed that Al12N12 and GaAl11N12 have a long recovery time and long-term desorption. Thus, it could not be used for sarin detection. On the other hand, SiAl11N12 demonstrated the short-term desorption suitable for sensor applications.
Conclusion
The interaction of sarin with the Al12N12, SiAl11N12, and GaAl11N12 nanoclusters was studied using DFT calculations to explore a new system for sarin detection. After optimization, adsorption energies were calculated at -38.36, -25.95, and -27.94 kcal mol-1 for Al12N12, SiAl11N12, and GaAl11N12, respectively. The electrical properties calculations indicated the electrical conductivity changes after adsorption of sarin are -3.04%, -24.64%, and -1.16% for Al12N12, SiAl11N12, and GaAl11N12, respectively. This variation can be considered as a signal of sarin detection. Thus, the SiAl11N12 nanocluster has a high sensitivity to the detection of sarin nerve agents. On the other hand, the recovery time calculation based on the transition theory confirmed that only the pristine SiAl11N12 showed a short recovery time of 9.93s s, demonstrating that sarin adsorption on that is reversible and more favorable. Therefore, it is concluded that the SiAl11N12 nanocluster can be used as a suitable sarin detector.
Acknowledgements
This article was derived from Ph.D. Degree thesis in the Islamic Azad University, Ardabil Branch.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
HOW TO CITE THIS ARTICLE
Mahdi Mohammad Alizadeh, Farshid Salimi, Gholamreza Ebrahimzadeh-Rajaei. Investigation of Adsorption Properties of Al12N12, SiAl11N12, and GaAl11N12 Nanoclusters for Sarin Detection: A Comparative DFT Study. Chem. Methodol., 2023, 7(3) 248-256


