Document Type : Short communication
Authors
- Milind V. Gaikwad 1
- Rahul D. Kamble 2
- Shrikant V. Hese 3
- Shuddhodan N. Kadam 4
- Ajay N. Abhore 5
- Sunil V. Gaikwad 6
- Ashok P. Acharya 7
- Bhaskar S. Dawane 8
1 Department of Chemistry, D.Y. Patil ACS College Pimpri, affiliated; Savitribai Phule Pune University, Pune (MS) India-411018
2 Department of Chemistry, Amruteshwar ACS, College, Vinzar, Pune (MS) India-412213
3 D.D. Bhoyar College of Arts and Science Mouda, Nagpur, 441104, MS, India
4 Department of Chemistry, VidnyanMahavidhyalaya, Sangola, Solapur (MS) India -413307
5 Department of Chemistry, PDVP College, Tasgaon, Sangli (MS) India -416312
6 Department of Chemistry, Savitribai Phule Pune University, Pune (MS) India-411007
7 Department of chemistry Mudhoji College, Phaltan- Satara(MS) India-415523
8 School of Chemical Sciences, SRTM University, Nanded (MS) India -431606
Abstract
The short, easy and total synthesis of Murrayanine (1), Mukonine (2), carbazole alkaloids were elaborated, based on a regioselective buchwald coupling of methyl 4-bromo-3-methoxybenzoate with aniline and successive transformation into the corrsponding carbazole alkaloids by oxidative coupling followed by cyclization of the phenyl and aryl rings.
Graphical Abstract
Keywords
Main Subjects
Introduction
The carbazole alkalods which are pharmacutical importance nitrogen containing heterocycle have been isolated from various natural sources. The exhaustive effort has been made toward the new discovery and construct of natural product. In the recent decade, numerous synthesis methods have been reported globally [1]. In the early 1982, Chakraborty et al. studied the roots of Murraya koenigii Spreng and isolated the carbazole alkaloids Mukoline and Mukolidine [2] (Figure 1).
Figure 1: Naturally occurring carbazoles
In India, the Murraya Koenigii is the best source for the isolation of the carbazole alkaloids such as murrayanine which is commonly called kadi patta leaf and used day to day life in kitchen [3]. The alkaloids display wide-ranging range of pharmaceutical activity such as antitumor, anti-inflammatory, anti-histaminic, antioxidant, light emitting properties [4]. Because of extensive use of distinctive skeleton, physicochemical properties and pharmaceutical activities as mentioned above, the introduction of novel, simple and efficient route to prepare the carbazole moiety has considerable attention in the scientist community. The numerous synthetic methods are present for the preparation of murrayanine (1), mukonine carbazole alkaloids [5-6].
The diverse synthetic reports available in the literature include Fischer indolization [7] the iron metal catalyzed oxidative coupling with cyclization of arylamine tricarbonyl(cyclohexadiene) complexes [8-9], oxidative cyclization in the palladium(II)-catalyzed [10], the thermal cyclization of 1-phenyl benzotriazole [11] cyclization of biarylnitrenes to carbazole [12] and some others methods. Recently, Banwell et al. described a new procedure for the preparation of Mukonine carbazole alkaloids with 66% yield via a Pd-Catalyzed oxidative C-C bond formation followed by reductive coupling reaction [13].Knoke et al. (1993) described the iron-promoted carbon-carbon, carbon-nitrogen bond formation followed by synthesis of koenoline, mukoeic acid, murrayafoliae A, murrayanine. murrayaquinone A, and mukonine natural product [14]. Subha etal. described the novel cross coupling reaction followed by reductive cyclization in the presence of triphenylphosphine that gives a range of carbazole alkaloids, including mukonine, murrayafoline A, mukoeic acid, clauszoline K, koenoline, murrayanine,glycoborine, mukoline, glycozolicine, mukolidine, and glycozoline alkaloids [15]. The natural product mukoline and mukolidine were produced by the reaction of aromatic amine with the exposure of palladium (II) acetate and copper (II) acetate in the pivalic acid [16].
As a researcher, always interested to report a novel methodology for the construction of bioactive heterocyclic compound [17-23]. In this paper, we have described a new method for the total synthesis of murrayanine (1), mukonine via construction of desire scaffold via Buchwald coupling followed by Pd(OAc)2 mediated oxidative coupling reaction to obtain desire molecule.
Material and methods
General
The experiment was performed in a dry glass apparatus and the required raw chemicals were bought from the national and international suppliers such as Spectrochem, Aldrich, Merck, Fisher and used directly. The dry reaction was carried out in the Argon gas atmosphere. The progress of reaction was checked on TLC. The synthesized compound purification was performed using column chromatography with Silica gel (60-120 Mesh) from Aura. The solvent was dried over A4 molecular sieves prior to use and the THF was dried over Na metal. The tetramethylsilane (TMS) used as internal standard at ambient temperature for the running of the 1H NMR, 13C NMR spectra over a Bruker 400, 500 MHz NMR machine. The FT-IR spectra were recorded over a Bruker- Perkin-Elmer model 683 B or 1605 spectrophotometer and absorptions were expressed in cm-1. All Buchi 501 apparatus was used for the recording of melting points and Boiling Points of pure compounds and are uncorrected.
Synthesis of methyl 4-bromo-3-methoxybenzoate (5)
To a solution of 4-bromo-3-methoxybenzoic acid, 6 (1,00 g, 1 eq) in methanol (15 ml) H2SO4 (20 µL, 0.15 eq) was added. The reaction mixture was blended to reflux for 5 h. After completion of the reaction, the solvent was removed under reduced pressure and the crude mass was mixed in DCM NaHCO3 and cold water. The organic layer was dried over sodium sulphate and concentrated under reduced pressure to afford the pure product 5 methyl 4-bromo-3-methoxybenzoate; white crystalline product, mp 54-56 °C.
Methyl 3-methoxy-4-(phenylamino)benzoate (3)
In a dry seal tube with magnetic stir bar, Pd2(dba)3, methyl 4-bromo-3-methoxybenzoate2 (g, mmol), aniline ( g, mmol) was added, followed by xantphos (g, mmol) Cs2CO3 (g, mmol) in a dry 1,4-dioxane, the seal tube was then evacuated/backfilled with argon 3x with stirring. The seal tube was stirred vigorously at 110 °C for 48h. After completion of reaction (monitored by TLC), the reaction mixture was diluted with 5 ml ethyl acetate and filter over sintered glass funnel, the organic solution concentrate under vacuum and purified using column chromatography to afford white crystal with 58% yield.
1HNMR (300MHz, CDCl3); δ: 3.88(s, 3H), 3.96 (s, 3H), 6.54 (br, 1H), 7.06 (t, J= 7.4 Hz, 1H), 7.19-7.26 (m, 3H), 7.34 (t, J= 8 Hz, 2H), .7.52 (d, J= 1.5 Hz, 1H), 7.59 (dd, J= 1.4 Hz & 8.1 Hz, 1H)
13CNMR (75 MHz, CDCl3), δ: 51.7, 55.7, 59.8, 110.5, 110.6, 119.9, 120.8, 123.0, 123.5, 129.4, 132.2, 140.6, 146.5, 167.1.
Synthesis of methyl 1-methoxy-9H-carbazole-3-carboxylate; Mukonine (2)
In a dry seal tube with magnetic stir bar was added Pd(OAc)2 and methyl 3-methoxy-4(phenylamino)benzoate 3in a 1,4-dioxane the seal tube was fluxed with argon 3x with stirring. The seal tube was blended vigorously at 100 °C for 16h. The progress of reaction was checked using TLC and after completion of reaction the mixture mix with 10 mL Ethyl acetate.
After completion of reaction (monitored by TLC), the reaction mixture was diluted with 10 mL ethyl acetate and filter over sintered glass funnel, the organic solution concentrate under vacuum and purified using column chromatography with ( ethyl acetate: hexane) to afford white solid.
Mukonine (5a): Yield (70%), mp 194-198 °C (lit. 4 mp 196 °C); 1H NMR (300 MHz, CDCl3): δ: 10.37 (s, D2O exchange, 1H), 8.34 (s, 1H), 8.06 (d, J=6.2 and washed with the saturated solution of
Hz, 1H), 7.61 (s, 1H), 7.51 (t, J = 9.1 Hz, J = 8.9 Hz, 1H), 7.40 (t, J = 8.8 Hz, J = 5.8 Hz, 1H), 7.09 (t, J = 6.2 Hz, 1H), 3.88 (s, 6H); 13CNMR (75 MHz, DMSO-d6): 167 δ 167.89, 146.01, 139.91 132.61, 126.02, 123.71, 123.83, 122.11, 120.22, 120.54, 116.21, 111.45, 107.23, 55.52, 55.01; The calculated HRMS for the molecular formula C15H13NO3 [M+ H]+, 256.0968 and observed 256.0970.
Synthesis of 1-methoxy-9H-carbazole-3-carbaldehyde; Murrayanine (1)
A solution of compound methyl 1-methoxy-9H-carbazole-3-carboxylate 0.500 mg in a 17 mL dry THF at -78 °C under the argon atmosphere di-isobutyl-aluminum hydride in THF was added drop wise and keep the vessel temperature below -65 °C. After completion of reaction, the reaction was quenched with 10 mL of 10% HCl, and extracted into 2 x 10 mL portions of ethyl acetate. The organic layer was also washed by using 10% HCl, solution and saturated sodium bicarbonate. The organic layer was dried over magnesium sulfate and concentration under reduced pressure gave desired 1-methoxy-9H-carbazole-3-carbaldehyde. Murrayanine (1):Colourless crystals, Yield (78%), mp 164-168 °C ; 1H NMR (400 MHz, DMSO) δ: 11.68 (s, 1H), 8.78 (s, 1H), 8.20 (d, J = 7.9 Hz, 1H), 8.03 (dd, J = 8.3, 5.5 Hz, 2H), 7.66 (d, J = 8.3 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 3.95 (s, 3H).
Result and Dissection
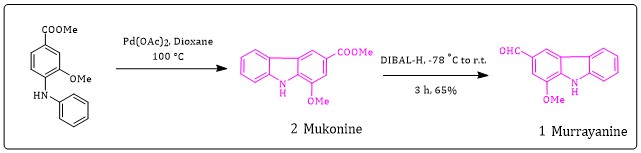
The retrosynthesis analysis of murrayanine (1), mukonine (2), is shown in Figure-1. Our retrosynthetic analysis for the synthesis of the target molecule 1 murrayanine (1) and mukonine (2) carbazoles are expected to be obtained via Buchwald–Hartwig coupling followed by Pd catalyzed C-C coupling reaction (Scheme 2). The key precursor 5 has been required for the construction of murrayanine and mukonine carbazole alkaloids.
Scheme 1: Retro synthesis of murrayanine (1), mukonine (2), carbazole alkaloids
With the help of synthetic pathway as shown in Scheme 2, the optimization of 1 involves carbon–nitrogen coupling with Buchwald coupling the catalyzed coupling reactions of amines with aromatic halides followed by the C-C oxidative coupling cyclization of the phenyl and aryl rings are the key steps. We started our strategy as per our proposed strategy (Scheme 1). The precursor 5 has been prepared form the etherification of 4-bromo-3-methoxybenzoic acid in MeOH/ H+ gives precursor 5. Then we moved towards the actual synthesis of desire precursor 3 from the Buchwald coupling with 5 and amine 4 by using Xantphos, Cs2CO3 and Pd(tris)3 [24] under the dry condition. At the beginning, we performed Buchwald coupling reaction by using various bases such as K2CO2, CsCO3, KOtBu. When the reaction was performed with the base K2CO3 at 100 °C to produce desired precursor, but very less yield, while in case of CsCO3, t-BuOK yield was moderate to good (Table 1).
Table 1: Examination of the Pd-catalyzed Buchwald coupling reaction
|
Sr /No |
Reagent |
Ligand |
Base |
Time |
Yield% |
|
1 |
Pd2(dba)3 |
BINAP |
K2CO3 |
24 |
20 |
|
2 |
Pd(OAc)2 |
Xanphose |
K2CO3 |
24 |
35 |
|
3 |
Pd2(dba)3 |
BINAP |
KOtBu |
24 |
28 |
|
4 |
Pd(OAc)2 |
Xanphose |
KOtBu |
24 |
42 |
|
5 |
Pd2(dba)3 |
BINAP |
Cs2CO3 |
24 |
30 |
|
6 |
Pd2(dba)3 |
Xanphose |
Cs2CO3 |
24 |
69 |
|
7 |
Pd(OAc)2 |
Xanphose |
Cs2CO3 |
24 |
38 |
Based on the above observation, we decided to examine the synthesis intermediate 3 via Buchwald coupling reaction. Initially, we examined the Pd-catalyzed Buchwald coupling reaction using Ligand BINAP and Xanphose in the presence of base K2CO3, KOtBu, Cs2CO3 (Table 1, entry 1). The treatment of 5 and 4 with Pd(OAc)2 Pd-catalyst in the presence of ligand Xanphose and base K2CO3, Cs2CO3 and KOtBu with dioxane solvent resulted in the formation of 3 with 35%, 38% and 42% yield (Table 1, entry 2, 4 and 7).
Scheme 2: Synthesis of murrayanine (1), mukonine (2), carbazole alkaloids
Based on the treatment of 5 and 4 with Pd2(dba)3Pd-catalyst in the presence of ligand BINAP, Xanphose and base K2CO3, KOtBu, Cs2CO3 with dioane solvent which resulted in the formation of 3 with 20%, 28%, 30% and 58% yield (Table 1, entry 1, 3, 5 and 6) from the above result, it can be concluded that the Buchwald coupling reaction occur by using Pd2(dba)3 reagent using Xanphose ligand with Cs2CO3 base in a 1,4-Dioxane solvent( table 1, entry 6). The desired compound 3 in our hand showed very good yield. Next, we embarked on the total synthesis of murrayanine (1) and Mukonine (2). The compound 3 were treated with Pd-catalyst and gave desired alkloids. Mukonine (2) was then prepared by oxidative coupling cyclization of the phenyl and aryl rings of precursor 3 initiated by stoichiometric amount of Pd(OAc)2 [25] in acetic acid solvent 7. Further, Mukonine (2) was converted to murrayanine (1) via reduction of ester group to aldehyde by using DIBAL-H in THF solvent with 78% yield.
Conclusion
We have described a new and concise synthesis method of Murrayanine (1) and Mukonine (2), by using the novel approach that involves a Pd-catalyzed Buchwald coupling followed by oxidative coupling cyclization of the phenyl and aryl rings using stoichiometric amount of Pd(OAc)2.
Acknowledgement
The authors are thankful to Department of Chemistry Dr. D.Y. Patil A. C. S. College, Pimpri, Pune, 411018, India, Savitribai Phule Pune University for providing laboratory facilities for this research. The author RDK is thankful to BOD, Savitribai Phule Pune University, Pune for ASPIRE Research Grant (18TCR000044).
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.