Document Type : Original Article
Authors
Department of Food Science and Technology, Faculty of Agriculture, Varamin-Pishva Branch, Islamic Azad University, Varamin, Iran
Abstract
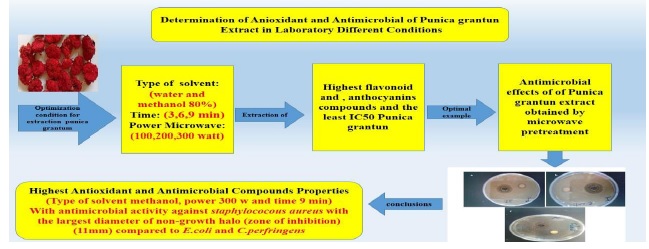
Microwave energy is non-ionizing radiation that causes the molecule to move and is a good way to extract compounds from plant extracts. This study aimed to investigate the effect of three independent variables including solvent type (water and methanol 80%), time (3, 6, and 9 minutes), and microwave power (100, 200, and 300 watts) on the flavonoids, anthocyanins, antioxidant properties (IC50) of the Persian Golnar plant or Punica granatum var. pleniflora (PGP) extract. According to results, simultaneous optimization of extraction conditions with 95.609% desirability in 300-watt microwave power, 9 minutes, and use of methanol solvent the flavonoid content was 5.7597 mg/g, anthocyanin content was 4.7983 µmol/g, and IC50 value was 6.5063 mg/ml. The highest average of minimum inhibitory concentration and minimum Bactericidal concentration of PGP extract were 1250 and 5000 µg/ml, respectively against Clostridium perfringens and Escherichia coli, and the better antimicrobial effect was against Staphylococcus aureus with the highest diameter of zone of inhibition (11 mm) compared to Escherichia and Clostridium perfringens. According to the results of this study, microwave pretreatment with optimized conditions can be used to extract significant amounts of phenolic and antimicrobial compounds of PGP extract as a rich source of antioxidants with minimal damage to its effective compounds.
Graphical Abstract
Keywords
Main Subjects
Introduction
Preservatives are compounds that are used to prevent the growth or elimination of harmful microorganisms to increase the shelf life of food. Today, the use of natural preservatives derived from medicinal plants is increasing due to its greater compatibility with the body, its flavor-producing volatile compounds, and antimicrobial and antioxidant properties [1].
The Persian Golnar is scientifically named Punica granatum var. pleniflora (PGP), from the Puniaceae family. Non-fruitful pomegranate flowers are used as an important medicinal plant in Fars province and some regions of northern Iran. All parts of the pomegranate tree are rich by tannins and have a relatively strong astringent effect, Therefore, in traditional medicine, it is used as a stypticity in a variety of clinical conditions, including hemorrhoids, nosebleeds, excessive uterine bleeding, and inflammation. Effective compounds in various parts of pomegranate, including leaves, bough, root, and fruit peel, pomegranate juice, and its seeds and flowers have antimicrobial properties as well as extensive antioxidant activity. Phenolic compounds are biological substances that are widely present in plants, especially in PGP, which are considered components of the human diet. Phenolic compounds of various plant groups, including flavonoids such as anthocyanins, flavonols, flavonoids, and non-flavonoids such as phenolic acids and lignins. PGP flowers due to their phenolic compounds such as gallic acid, ursolic acid, and terpenoids such as maslinic acid and acetic acid has antioxidant and antibacterial properties [2-4]. The most important chemical compounds in the peels and roots of pomegranate are Ellagic acid, Ellagic tannins, Gallic acid, and Anthocyanins as well as piperidine alkaloids which the structure and phenolic nature of this compound cause its strong antioxidant activity [5]. The first step is to perform antioxidant tests and use plants in the plant extract industry. There are several methods to extract these compounds. One of these methods is the maceration method, which is a time-consuming method with high solvent consumption and environmental pollution. Therefore, newer methods such as microwave extraction and extraction of effective plant compounds are used today [6].
Microwaves, electromagnetic radiation with a frequency of 0.3-300 GHz, with the rapid rise in temperature and the destruction of cells containing essential oils in a very short time causes the release of essential oils (Vian et al., 2008) Extraction by microwaves is based on the absorption of microwave energy by polar molecules of the chemical compounds. In Microwaves method, due to the special design of the device and the location of the condenser and collector parts at the bottom of the microwave oven, gravity is used to move the essential oil towards the condenser. This design system significantly reduces process time compared to conventional systems. The main purpose of this study was to investigate the effect of the microwave extraction method on the content of flavonoid compounds, anthocyanins, antioxidant, and antimicrobial activity of PGP extract.
Materials and Methods
Preparation of PGP
The 500 gram of flowers of PGP were collected from pomegranate orchards (Saveh, Iran), in May/July 2020. The samples were authenticated by a professional herbalist using a voucher specimen (KF1634-1) deposited in the herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences. Then, to reduce the humidity of flowers to less than 10%, the samples were placed in the UF55 / UN oven, Mommert company (Germany) at 45 °C for 24 hours. After drying the samples were reaching a constant weight, using the ML-320P model mill, Pars Khazar Company (Iran), the sample was milled in the form of flour granules using 16 meshes. The resulting powder was stored for testing in polyethylene containers in a dry place at 25 ° C. Escherichia coli (PTCC: 1338), Staphylococcus aureus (PTCC: 143), and Clostridium perfringens (PTCC: 1766) were prepared as lyophilized from the microbial collection of Iran Scientific and Industrial Research Organization.
Extraction by microwave method
According to Table 1, pigment extraction from PGP according to Full factorial design with three independent variables of solvent type (water and methanol 80%), time (in three levels of 3, 6, and 9 minutes), and power (in three levels of 100, 200 and 300 Watts) was done. In this method, 5 g of PGP powder with a ratio of 1: 5 (solvent (ml): plant material (g) was mixed with each of water and methanol 80% solvents. The resulting mixtures were then exposed to microwaves with powers (100, 200, and 300 watts) at different times (3, 6, and 9 minutes). The extract was then isolated by Whatman No. 1 filter paper and the filtered of extract was concentrated by IKA rotary evaporator, RV10 DS99 model (Germany), at 50 °C to 60 Brix, and then dried in an oven at 40 °C until complete drying [7].

Measuring the total amount of flavonoid compounds
The total amount of flavonoid compounds in PGP extract was measured by a colorimetric method. The amount of 0.5 ml of the extract was dissolved in 1.5 ml of methanol in a test tube, and then 0.1 ml of 10% aluminum chloride and 0.1 ml of 1 M potassium acetate solution were added. Finally, 2.8 ml of distilled water was added to them and kept at room temperature for 30 min and then the absorption of the mixture was read at 415 nm by spectrophotometer. Quercetin was used as a standard with different concentrations (6.25, 12.5, 25, 50, and 80 ppm) to draw the calibration curve. The content of flavonoids was expressed in milligrams equivalent to quercetin per gram of dry sample [8].
Measuring the total amount of anthocyanin compounds
To determine the content of anthocyanin compounds, the pH change method was used. Firstly, 2 ml of PGP extract with 25 ml of a buffer solution with pH = 1, which included a mixture of 0.2 M potassium chloride and 0.2 Molarity chloric acid, and then another 2 ml of an extract with buffer solution with pH= 4.5 which included a mixture of 1 M sodium acetate and 1 M hydrochloric acid was reached to a volume of 25 ml and the absorbance of the samples was read at 510 nm. The concentration of anthocyanins was calculated by Equation 1 below.
Equation 1:
Cmg/l = ×DF
The numbers 484.82 and 24825 are the molecular weight and the molar absorption coefficient of the cyanidin-3-glucoside molecule at 510 nm in the buffer solution, respectively. DF is also a dilution factor [9].
Evaluation of DPPH free radical scavenging activity
The ability to scavenge the free radical of PGP was measured by decolorizing of DPPH ethanolic solution. 2-2-Diphenyl-1-picryl hydroxyl is a stable radical compound with a purple color that is reduced to yellow diphenylpicryl hydrazine by electron reduction or hydrogen donor elements (antioxidant compounds). In this method, DPPH was used as a reagent for stable radical compounds. Thus, 50 μl of different concentrations of essential oil in ethanol was added to 5 ml of 0.004% DPPH solution in ethanol. After 30 minutes of incubation at room temperature, the light absorption of the samples was read at 517 nm against the control, and the percentage of free radical scavenging was calculated using Equation 2 below [10].
Equation 2:
DPPH = (sample percentage absorption-control percentage absorption) / (control percentage absorption) X 100%
For better evaluation of anti-radical activity, IC50 factor was used, which indicates the concentration percentage of PGP extract, which can neutralize 50% of DPPH free radicals. To calculate IC50, first, the calibration curve of inhibition power in terms of (mg/ml) was plotted and the graph line equation (y = 0.013x + 8.22) was obtained, and by substituting 50 in the y-axis, the IC50 value was calculated from the x-axis.
Microbial tests
The antimicrobial effect of optimal treatment of microwave extraction pretreatment with the highest antioxidant properties was investigated by MIC (Minimum Inhibitory Concentration), MBC (Minimum Bactericidal Concentration) and well and disc zone of inhibition on Escherichia coli, Staphylococcus aureus, and Clostridium perfringens bacteria. To evaluate the antimicrobial properties of the mentioned extracts, 96-well microplates were used. In each wells, the first 95 μl of Müeller-Hinton Broth culture medium and 5 μl of bacterial suspension, equivalent to 0.5 McFarland, tube were added. Then 100 μl of successive dilutions of extract were added to each well. After mixing the samples with a shaker (20 seconds at 300 rpm), they were incubated for 37 hours at 37 ° C, and after this period, the turbidity rate was read and recorded at a wavelength of 540 nm by ELISA (American Biotech Company Model ELX 800) and if there was no turbidity, the minimum inhibitory concentration of bacterial growth (MIC) was determined. Then, samples from wells without turbidity were passed on Müeller Hinton agar culture medium and colony count was performed by successive dilution method. The first tube with a reduction in bacterial growth rate greater than one-thousandth compared to the zero time of the control tube was determined as the Minimum Bactericidal Concentration (MBC) [11].
Statistical Analysis
Designs of treatments, analysis, and optimization of test results were performed using the full factorial design method in Minitab 16 software. To compare and evaluate the antimicrobial properties of the optimal sample, one-way analysis of variance Duncan's in Minitab 16 software was used.
Result and Discussion
The extraction efficiency of phenolic compounds depends on many factors such as time, temperature, particle size, sample matrix porosity, solvent type, solvent concentration, pH, sample to solvent ratio, and solvent diffusion coefficient in the sample [12].
The power of the microwave depends entirely on the temperature and time of extraction. Increasing the power of the microwave along with the extraction temperature causes the cell to rupture rapidly and, therefore, increases the content of impurities in the extracts [13]. The use of microwaves increases the extraction efficiency of biological compounds compared to other common methods, which is due to the interaction of microwaves with polar molecules, leads to heat and internal pressure of solids [14].
The content of flavonoids in PGP extracted by microwave method
The experimented and predicted of flavonoid content of PGP extracted by microwave pretreatment method has been reported in Table 2. Based on the results of different extraction conditions (microwave power, time, and type of solvent), microwave pretreatment method had a significant effect on the flavonoid content of PGP extracted. The content of flavonoids ranged from 3.05 to 5.903 mg/g. The results showed that increasing microwave power (from 100 to 300 watts), extraction time (from 3 to 9 minutes), and the use of methanol solvent compared to the use of aqueous solvent had a significant effect (P≤0.05) in increasing the content of flavonoids. The highest flavonoid content of 5.903 mg/g in 300-watt microwave power was obtained in 9 minutes with methanol solvent and the lowest flavonoid content of 3.05 mg/g in 100-watt microwave power was obtained in 3 minutes in a water solvent. It was shown that the use of methanol solvent compared to water solvent, extraction time from 3 to 9 minutes, and microwave power from 100 to 300 watts caused the extraction of more flavonoid compounds from PGP. Flavonoids are polyphenolic compounds that are mainly found in plants. And appear as potent antioxidant and anti-radical compounds [15]. Polyphenols may be present along with sugars, proteins in plant tissues, and create polymerized derivatives, which complicate the extraction process [16]. Differences in the polarity of methanol and water have led to higher levels of flavonoid compounds extracted from PGP by Methanol solvent. The polarity of the solvents used plays a key role in increasing the solubility of these compounds [18].

The basic principle of the microwave is based on the direct effect of the microwave on ionic conductivity of molecules, or their polar rotation. Polar molecules, such as water, have positive and negative charges at opposite ends and rotate in an attempt to align with the electric field formed as the power of the microwave increases [19]. Molecular displacement and friction between molecules create magnetic energy which is converted into heat energy and increasing the temperature of the matrix causes cell destruction and facilitates the exit of compounds from the cell [20] as well as increasing the mass transfer of soluble compounds which increases solvent efficiency. The microwave absorbed by the sample generates heat. The Heat leads to evaporate the sample water and increases the pressure of the sample and destroys the cell wall. In this way, the active compounds are easily removed from the sample. Therefore, with increasing microwave power, cell wall breakage occurs faster and better [21]. As the temperature increases, the internal pressure of cell wall degradation accelerates, and the exit of compounds from the tissue into the solvent increases [22, 23].
Beejmohun et al., (2007) reported that the use of microwaves increases the extraction efficiency of biological compounds compared to other common methods. Since the energy in the microwave is very high, the conditions for extracting biological compounds by the microwave need to be optimized [14]. In a similar study, Gallo et al. (2010) reported that the use of microwaves significantly reduced the extraction time [24]. Ince et al. (2013) reported that the amount of total phenol extracted from lemon balm by microwave method under studied conditions was equal to 24.64 mg GAE/g dry matter [25].
The amount of anthocyanin in PGP extract extracted by microwave method
The amount of anthocyanin in PGP extract extracted by microwave pretreatment method in different conditions has been reported by the method tested and predicted in Table 2. Different extraction conditions (microwave power, time, and type of solvent) had a significant effect on the content of anthocyanin in PGP extract extracted by microwave pretreatment. The content of anthocyanin ranged from 2.818 to 4.970 µmol/g. The results showed that increasing microwave power (from 100 to 300 watts), extraction time (from 3 to 9 minutes), and the use of water solvent compared to the use of methanol solvent had a significant effect in increasing the amount of anthocyanin (P≤0.05). The highest content of anthocyanin extraction was performed using water solvent, extraction time 9 minutes, and microwave power of 300 watts. Water as a solvent for extraction creates a polar environment in which high-polarity compounds are mostly extracted [26]. The greater the power of the microwave, the greater the breakdown and disintegration of the cell walls and the greater the possibility of releasing their contents, and the time factor increases the duration of the mass transfer, so the upward trend in the content of anthocyanin extracted from PGP seems to be quite reasonable with increasing time. The stability of anthocyanins depends on several factors such as pH, oxygen concentration, temperature, light, solvent type, and the presence of other compounds. Anthocyanins were stable at low pH [27]. Accordingly, Chen et al., (2007) reported that the optimal conditions for extracting anthocyanins from red raspberries using the microwave method were at 55°C, the irradiation time of 12 minutes, and the microwave power of 366 watts [28].
Elez Garofulić et al. (2020) in their study of the effect of microwave extraction on the separation of anthocyanins and phenolic acids from Pistacia lentiscus reported that the difference in microwave power in the extraction of these compounds was significant and for phenolic compounds and anthocyanins 400-watt power was most effective. And compared to conventional extraction methods, microwaves have shown higher performance in extraction [29].
Radical inhibition activity in PGP extract extracted by microwave method
The ability of a substance to neutralize free radicals is expressed by a factor of IC50, which indicates the content of the sample required to inhibit 50% of free radicals. Thus, less IC50 indicates more antioxidant potential (low concentration of the sample can prevent a large number of free radicals) [30]. The results of comparison between the IC50 of PGP extracted by microwave method under different conditions by the tested method with the predicted one with different solvents in different powers and different times are presented in Table 2. According to the results, different extraction conditions (microwave power, time, and type of solvent) had a significant effect on the IC50 of PGP extracted by the microwave method. So that, the amount of IC50 of PGP extracts varied in the range of 6.499 to 38.256 (mg /ml). The results showed that increasing microwave power (from 100 to 300 watts), extraction time (from 3 to 9 minutes), and the use of methanol solvent relative to water, had a significant effect on reducing the content of IC50 of PGP extract (P≤0.05). The results showed that the lowest level of IC50 (6.499 mg/ml) at microwave power of 300 watts, the extraction time of 9 minutes, and the use of methanol solvent relative to water had a significant effect (P≤0.05) on reducing the IC50 of the PGP extract. DPPH radical is widely used to evaluate the trapping ability of free radicals in various samples. DPPH is a free radical with a central nitrogen atom that changes color from purple to yellow as it reduces and produces a stable DDPH-H molecule. The DPPH radical is absorbed at 512 to 528 nm, but as soon as it is reduced by an antioxidant, the absorption decreases. The antioxidant activity of the samples is expressed as the disappearance of the purple color and finally to the yellow color. [31] Among the plant compounds that have antioxidant properties, phenolic compounds which are widely distributed in many plants. Several studies have shown that plant extracts are rich in flavonoids and phenolic compounds and due to their antioxidant properties, they protect of cells. The antioxidant properties of these compounds are mainly due to their reducing power and chemical structure, which enables them to neutralize free radicals, form complexes with metal ions, and extinguish single and triple oxygen molecules [32].
Akbari et al., (2020) reported that under saponin extraction conditions, phenolics and antioxidant compounds extracted from fenugreek using 572 watts of microwave power, 87.36% ethanol, and a time of 2.84 minutes, the mass ratio was 0.09 grams per liter [33]. According to the results of Alara et al. (2019), the best conditions for microwave process parameters with the highest yield (61.25%) were extraction of total phenolic content and antioxidant activity of fruit peel extract from Phaleria macrocarpa for 1 minute, 80 °C and, 300 g weight [34].
Polynomial model of extracted PGP extract in different conditions
Analysis of variance was performed on flavonoids of this quadratic polynomial model, the results of which are shown in Table 3. As can be seen in this table, the value of the coefficient of determination of this model was 99.11 and the corrected coefficient of determination was 98.33, which indicates the good fit of the model to the experimental data.
Analysis of variance was performed on anthocyanins of this quadratic polynomial model, the results of which are shown in Table 3. As can be seen in this table, the value of the coefficient of determination of this model was 97.10 and its corrected coefficient of determination was 94.51, which indicates a good fit of the model to the experimental data.
Analysis of variance was performed on IC50 of this quadratic polynomial model, the results of which are shown in Table 3. As can be seen in this table, the value of the coefficient of determination of this model was 99.73 and the corrected coefficient of determination was 99.49, which indicates the good fit of the model to the experimental data.

Results of analysis of variance of response level model
Phenols are a class of compounds consisting of one or more hydroxyl groups bounded to an aromatic (nonpolar) ring, and this spatial structure distinguishes phenols based on their According to Table 4, the linear effects of all three variables of microwave power, microwave time, and solvent type, the quadratic effect of microwave power, and interaction (power × microwave time) on the flavonoid content of PGP extract were significant (p≤0.05). But the quadratic effects of solvent type and microwave time and interactions (solvent type × microwave power) and (time × solvent type) on flavonoid content of PGP extract were not significant (P> 0.05). According to the amount of factor F, the linear effect of microwave power and then the linear effect of microwave time had the greatest effect on the flavonoid content of PGP extract.
According to Table 4, the linear effects of all three variables of microwave power, microwave time, solvent type, and interaction (microwave power × time) on the content of anthocyanin in PGP extract were significant (p≤0.05). But the quadratic effects of solvent type, extraction time, and microwave power and interactions (solvent type × microwave power) and (solvent type ×extraction time) on anthocyanin content of PGP extract were not significant (P> 0.05). According to the amount of factor F, the linear effect of microwave power, and then the linear effect of microwave time had the greatest effect on the content of anthocyanin of PGP extract.
According to Table 4, the linear effects of power, microwave time, solvent type, and the quadratic effect of microwave power on IC50 (mg/ml) of PGP extracted by microwave method were significant (p≤0.05). However, the quadratic effects of solvent type and extraction time and the interaction effects of microwave power, extraction time, and solvent type on IC50 of PGP extracted by microwave method were not significant (P> 0.05). According to the amount of factor F, the linear effect of microwave power, and then the linear effect of microwave time had the greatest effect on the content of antioxidant compounds of PGP extract.

Single optimization conditions for flavonoids, anthocyanins, and IC50 in PGP extract extracted by microwave method
Figure 1a. shows the optimal conditions to maximize the content of flavonoids in PGP extract isolated by microwave method. According to this predicted shape, the maximum content of flavonoids in PGP extract is 5.7607 mg/g with 95.10% desirability, in terms of extraction time of 9 minutes, microwave power of 300 watts, and the use of methanol solvent. Optimal conditions for extracting the maximum content of predicted flavonoids were practically applied in the laboratory and the flavonoid content was 5.803 mg/g, which was not significantly different from the content of predicted phenol by the response level method (P> 0.05).
Figure 1b. shows the optimal conditions for the maximum content of anthocyanin in the PGP extract isolated by the microwave method. According to this predicted figure, the maximum content of anthocyanin in PGP extract is 4.9474 µmol/g with 98.969% desirability, under conditions of 9 minutes’ extraction time, microwave power of 300 watts, and the use of water solvent. Optimal conditions for extracting the maximum content of predicated anthocyanin were practically applied in the laboratory and the anthocyanin content was 4.876 µmol/g, which was not significantly different from the predicted phenol content by the response level method (P> 0.05).
Figure 1c. shows the optimal conditions to maximize the antioxidant properties of PGP extract isolated by the microwave method. According to this figure, it was predicted that the lowest IC 50 level of PGP extract, in other words, the maximum content of antioxidant compounds of PGP extract that can inhibit free radicals, is (6.5063 mg /ml) with 99.978% desirability, in time condition of 9 minutes, the microwave power of 300 watts, and the use of methanol solvent. Optimal conditions of maximum antioxidant compounds of PGP extract or the lowest content of predicted IC50 (mg/ml) was practically applied in the laboratory and the content of IC50 (mg/ml) was 6.609, which was not significantly different from the predicted IC 50 by response level method (P> 0.05).

Figure 1: Single optimization diagram of flavonoid content, anthocyanin, and IC50 in PGP extract extracted by microwave method a) flavonoid content, b) anthocyanin, c) IC50
Simultaneous optimization of flavonoids, IC 50 (mg/ml), and anthocyanin of PGP extract extracted by microwave method under different conditions
Figure 2 shows the simultaneous optimization of flavonoids, IC50, and anthocyanin of PGP extract extracted by microwave method under different conditions. Optimization of extraction conditions of PGP extract by microwave pretreatment method to achieve the highest content of flavonoids and anthocyanins, the lowest amount of IC50 simultaneously with 95.609% desirability at 9 min extraction time, 300-watt microwave power, and solvent use methanol was predicted. According to the results of Figure 2, in the mentioned conditions, the flavonoid content was 5.7597 mg/g, the anthocyanin content was 4.7983 µmol/g, and the IC50 content was 6.5063 mg/ml. Predicted optimal condition was practically applied in the laboratory and its IC50 content was 6.609 mg/ml, anthocyanin content was 4.876 µmol/g, and flavonoid content was 5.533 mg/g, which was not significantly different from total predicted IC50, anthocyanins, and flavonoids by the response level method (P> 0.05).

Figure 2: Simultaneous optimal conditions of flavonoids, IC 50 (mg/ml), and anthocyanin of PGP extract extracted by microwave method in different conditions
Antimicrobial effects of PGP extract extracted by microwave method
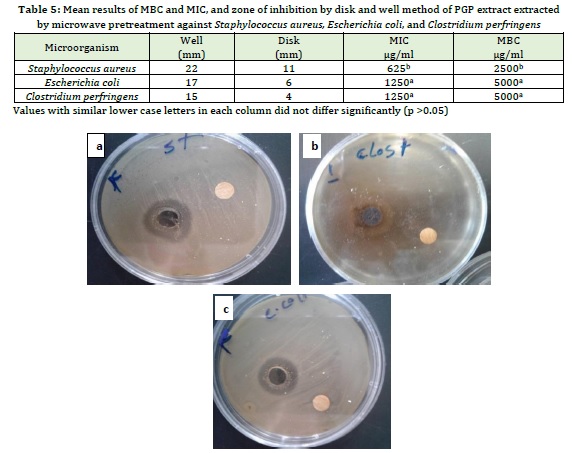
Predicted optimal treatment by microwave pretreatment method, which had the highest antioxidant properties, was produced in practice and after confirming its antioxidant properties, the antimicrobial effect of optimal treatment by MIC and MBC methods and zone of inhibition by both disk and well methods on Escherichia coli, Staphylococcus aureus, and Clostridium perfringens microorganisms were studied. Table 5shows the minimum inhibitory concentration and the minimum bactericidal concentration of the optimal treatment predicted by microwave pretreatment against Staphylococcus aureus, Escherichia coli, and Clostridium perfringens. The results showed that there was no significant difference between the minimum inhibitory and Bactericidal concentrations of PGP extract extracted by microwave pretreatment against Clostridium perfringens and Escherichia coli (p>0.05). The lowest mean values of MIC and MBC of PGP extracted by microwave pretreatment were 625 and 2500 µg/ ml against Staphylococcus aureus, respectively, and the highest mean values of MIC and MBC of PGP extracted by microwave pretreatment were 1250 and 5000 µg/ml, against Clostridium perfringens and Escherichia coli respectively. Finally, the optimal treatment predicted by microwave pretreatment had a better antimicrobial effect against Staphylococcus aureus with the largest diameter of zone of inhibition (11 mm) compared to Escherichia coli and Clostridium perfringens.
Antimicrobial compounds in herbs and spices are typically associated with phenolic compounds that have a hydroxyl (OH) group and are responsible for the antimicrobial properties of compounds such as thymol and carvacrol found in medicinal plants. The hydroxyl group in the phenolic compounds binds to the active part of the enzymes and prevents their metabolism. There is an important synergist between carvacrol and its precursor para-cymene So that the para-cymene first swells the cell membrane of the microorganism. Subsequently, more carvacrol enters the cell, and eventually the effect of carvacrol causes the destruction of the microorganism [35]. Another mechanism that may occur is that these compounds bind to phospholipids in the cell membrane, reducing selective permeability and increasing membrane permeability. In this case, cellular components are removed from the cell, and energy metabolism is impaired. There is also a change in the uptake of nutrients by the microbial cell and the transfer of electrons in it and the synthesis of genetic material [35]. Excessive acidification in plasma membrane interphase, which results from the dissociation of phenolic acids, alters the potential of the cell membrane and leads to increased permeability. This mechanism can explain the differences in sensitivity to phenolic acids between different pathogenic microorganisms [36,37]. Gram-positive bacteria lack an outer membrane, which allows phenolic compounds to spread more easily into the cell than gram-negative bacteria. In other words, the outer membrane of gram-negative bacteria is a barrier against excessive acidification of the membrane, and this can indicate the difference between the different resistances of gram-negative bacteria [36]. Antimicrobial properties Pomegranate peel extract was higher in gram-positive bacteria than gram-negative bacteria, which is consistent with the results of this study, so that the gram-positive Clostridium perfringens bacterium was the most sensitive bacterium to PGP extract [38]. The results are consistent with the results obtained by Dahham et al., (2010), which studied the antimicrobial effect of pomegranate peel extracts against B. cereus, E. coli, S. aureus, and P. aeruginosa.
Sodzim et al., (2012) reported in their study that the use of black pepper extracts, garlic, and their combination in pork at room temperature of 23 ᵒC significantly reduced aerobic bacteria.
Conclusion
Microwave extraction is a suitable method to reduce solvent consumption, short extraction time, relatively high recovery, good production. Because the plant matrix contains significant amounts of water, it absorbs microwave energy and causes cell destruction, which facilitates the extraction process. Microwave power depends entirely on the extraction time; therefore; optimizing the type of solvent, the time, and power of the microwave is essential to prevent the degradation of effective compounds. The results obtained from the present study indicate the presence of significant amounts of flavonoid compounds, anthocyanins, antioxidant properties, and antimicrobial properties in a special extract of PGP plant by microwave method. According to the practical results of the optimized conditions in the laboratory, the amount of IC50 (mg/ml) was 6.609, the amount of anthocyanin was 4.876 µmol/g, and the content of flavonoids was 5.803 mg/g. In general, microwave pretreatment with methanol solvent, time of 9 minutes, and power of 300 watts were recognized as the most effective parameter in the extraction of phenolic compounds of the extract.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID
Leila Nateghi:
https://www.orcid.org/0000-0001-8937-8728
HOW TO CITE THIS ARTICLE
Ali Reza Maleki, Leila Nateghi, Peyman Rajai, The Effect of Microwave Pretreatment on the Extraction Rate of Flavonoid, Anthocyanins, Antioxidant Compounds and Antimicrobial Activity of Punica Granatum Var. Pleniflora (Persian Golnar). Chem. Methodol., 2022, 6(4) 280-292
DOI: 10.22034/CHEMM.2022.316547.1399
- Tajkarimi M.M., Ibrahim S.A., Cliver D.O., Food Control., 2010, 21:1199 [Crossref], [Google scholar], [Publisher]
- Cushnie T.P.T., Lamb A.J., J. Antimicrob. Agents, 2005, 26:343 [Crossref], [Google scholar], [Publisher]
- Jurenka J., Med. Rev., 2008, 13:128 [Google scholar], [Publisher]
- Zargari A., Medicinal Plants. 1996, 7th Edition. Tehran: Tehran University, 372 [Publisher]
- Salahvarzi Y., Tehranifar A., Jahanbakhsh V., J. Med. Aromatic Plants, 2011, 27:47 [Crossref], [Google scholar], [Publisher]
- Wang L., Weller C.L., Trends Food Sci. Technol., 2006. 17:300 [Crossref], [Google scholar], [Publisher]
- Pan X., H Liu., G. Jia, Y.Y. Shu., Eng. J., 2000. 5:173 [Crossref], [Google scholar], [Publisher]
- Chang C.C., Yang M.H., Wen H.M., Chern J.C., Food Drug Anal., 2002, 10:178 [Crossref], [Google scholar], [Publisher]
- Rapisarda P., Fanella F., Maccarone E., Agric. Food Chem., 2000, 48:2249 [Crossref], [Google scholar], [Publisher]
- Yao X.H., Zhang D.Y., Zu Y., Fu Y., Luo M., Gu C.B., Li C.Y., Mu F.S., Efferth T., Crops Prod., 2013, 49:247 [Crossref], [Google scholar], [Publisher]
- Harikrishnan R., Nisha R.M., Balasundaram C., Aquaculture, 2003, 221:41 [Crossref], [Google scholar], [Publisher]
- Wang L., Weller C.L., Trends Food Sci. Technol., 2006. 17:300 [Crossref], [Google scholar], [Publisher]
- Camel V., Trends Analyt. Chem., 2000, 19:229 [Crossref], [Google scholar], [Publisher]
- Beejmohun V., Fliniaux O., Grand E., Lamblin F., Bensaddek L., Christen P., Kovvensky J., Fliniaux M., Mensnard F., Anal., 2007, 18:275 [Crossref], [Google scholar], [Publisher]
- Fathiazad F., Ahmadi-Ashtiani H.R., Rezazadeh S., Jamshidi M., Mazandarani M.A.S.O.U.M.E.H., Khaki A.R.A.S.H., Med. Plants, 2010, 9:177 [Google scholar], [Publisher]
- Pascual-Teresa S.D., Sanchez-Ballesta M.T., Rev., 2008, 7:281 [Crossref], [Google scholar], [Publisher]
- British Pharmacopoeia, Medicines and Healthcare Products Regulatory Agency (MHRA). 2009, 6939 [Publisher]
- Barba A.A., Calabretti A., d'Amore M., Piccinelli A.L., Rastrelli L., LWT-Food Sci. Technol., 2008, 41:1919 [Crossref], [Google scholar], [Publisher]
- Aspé E., Fernández K., Cienc. tecnol., 2011, 13:243 [Crossref], [Google scholar], [Publisher]
- Mandal, V., Mohan, Y., Hemalatha, S., Rev., 2007, 1:7 [Google scholar], [Publisher]
- Khalili M.a., Ebrahimzadeh M.A., Mazandaran Univ. Med. Sci., 2015, 24:188 (Persian) [Google scholar], [Publisher]
- Korukluoglu M., Sahan Y., Yigit A., Food Saf., 2008, 28:76 [Crossref], [Google scholar], [Publisher]
- Camel V., Trends Analyt. Chem., 2000, 19:229 [Crossref], [Google scholar], [Publisher]
- Gallo M., Ferracane R., Graziani G., Ritieni A., Fogliano V., Molecules. 2010, 15:6365 [Crossref], [Google scholar], [Publisher]
- Ince A.E., Sahin S., Sumnu S.G., J. Agric., 2013. 37, 69 [Google scholar], [Publisher]
- Al-Zoreky N.S., J. Food Microbiol., 2009, 134:244 [Crossref], [Google scholar], [Publisher]
- Rubinskiene M., Jasutiene I., Venskutonis P.R., Viskelis P., Chromatogr. Sci., 2005, 43:478 [Crossref], [Google scholar], [Publisher]
- Chen F., Sun Y., Zhao G., Liao X., Hu X., Wu J., Wang Z., Sonochem., 2007, 14:767 [Crossref], [Google scholar], [Publisher]
- Elez Garofulić I., Kruk V., Martić A., Martić I., Zorić Z., Pedisić S., Dragović S., Dragović-Uzelac V., Foods, 2020, 9:1556 [Crossref], [Google scholar], [Publisher]
- Xiao W., Han N., Shi B., Purif. Technol., 2008, 62:614 [Crossref], [Google scholar], [Publisher]
- Khalili M.a., Ebrahimzadeh M.A., Mazandaran Univ. Med. Sci., 2015, 24:188 (Persian) [Google scholar], [Publisher]
- Ahmadi F., Kadivar M., Shahidi M., Food chem., 2007, 105:57 [Crossref], [Google scholar], [Publisher]
- Akbari S., Nour A.H., Yunus R.M., IOP Publishing. Conference Series: Materials Science and Engineering, 2020, 736:022 [Google scholar], [Publisher]
- Alara O.R., Abdurahman N.H., Abdul Mudalip S.K., Olalere O.A., Jundishapur J. Nat. Pharm. Prod., 2019, 14:e57620 [Crossref], [Google scholar], [Publisher]
- Chitra K., Venkatesh R., Dhanalakshmi K., Sharavanan PT., Bali-Sasikumar C., Karthikeyani-Vijayakumari K., J. Curr. Microbiol. App. Sci., 2018, 7:379 [Crossref], [Google scholar], [Publisher]
- Cueva C., Moreno-Arribas M.V., Martín-Álvarez P.J., Bills G., Vicente M.F., Basilio A., Rivas C.L., Requena T., Rodríguez J.M., Bartolomé B., Microbiol., 2010, 161:372 [Crossref], [Google scholar], [Publisher]
- Lu X., Wang J., Al-Qadiri H.M., Ross C.F., Powers J.R., Food Chem., 2011, 129:637 [Crossref], [Google scholar], [Publisher]
- Al-Zoreky N.S., J. Food Microbiol., 2009, 134:244 [Crossref], [Google scholar], [Publisher]