Document Type : Original Article
Authors
Department of Chemistry, College of Science, University of Baghdad, Baghdad, Iraq
Abstract
The microemulsion approach was used to create three new nano nanocomposites: Lead oxide (LO)/PbO, lead iron oxide (LIO)/PbO-Fe2O3 and lead iron oxide polypyrrole (LIOPYY)/PbO-Fe2O3-polypyrrole. X-ray diffraction (XRD), Raman, field emission scanning electron microscopy (FESEM), Brunauer–Emmett–Teller (BET) surface area, vibrating-sample magnetometer (VSM), and thermal gravimetric analysis were used to describe the nanocomposites (TGA). LO and LIO composites have an orthorhombic crystal structure, whereas LIOPYY nanocomposites have a monoclinic crystal structure, according to XRD studies. The Scherrer equation was used to calculate the crystallite size, which was a high value in LO and a low value in LIOPYY. A considerable shift in the composite structure was discovered using Raman analysis, confirming the formation of nanomaterials. The AC conductivity measurements revealed that LO has a lower conductivity than LIO, whereas LIOPYY has a greater conductivity. LIO exhibits higher Ms Values when measured using the VSM method.
Graphical Abstract
Keywords
Main Subjects
Introduction
Organo-inorganic hybrid materials can combine a range of qualities including selectivity and activity [1-5]. They improve physicochemical properties, such as increased CO2 adsorption and conductivity. They also have enriched types of active sites, maximized active site exposure, and manipulated reaction pathways [6]. Nanoparticles (NPs) have a larger surface area to volume ratio [7] and a variety of intriguing features, including data storage capacity [8], catalysts [9], sensing to optics [10–12], and antimicrobial activities [13,14]. Microelectronics [15], Photovoltaic devices [16], gas sensors [17,18], corrosion-protecting devices [19], air purification and water purification [20,21] are only a few of the applications for metal oxide [22].
Hematite (Fe2O3) is an n-type semiconductor oxide with a direct band gap of 2.1 eV. It is widely employed in the catalysis, domains of solar energy conversion, gas sensing, purification, lithium-ion batteries, water splitting and biomedicine, and so on as a low-cost semiconductor material with a narrower bandgap (2.2 eV) [23-28].
Conductive polymers have received copious attention in the last three decades because of their exceptional characteristics and possible uses [29]. Nanocomposites consisting of metal oxides and conductive polymers are gaining popularity in various combinations. Metal oxides have been proven to successfully improve the electrical, optical, mechanical, and dielectric properties of several conductive polymers in previous research [30, 31].
Polypyrrole has good electrical conductivity, ambient stability, biocompatibility, and polymerization over various metal oxides [31]. For the preparation of nanomaterials, a variety of approaches has been devised. Mechanical milling, for example, is one of these procedures [32]. Chemical approaches include chemical reduction [33], photochemical reduction [34], electrodeposition [35], hydrothermal [36], and sol-gel synthesis [37,38]. Microemulsion has shown to be one of the most versatile and reliable chemical approaches for gaining control over the process. It has been found that increasing the nanoparticle size results in nanoparticles with a limited size distribution [39]. The purpose of the research is to synthesize new nanocomposites improve the electrical, magnetic, and surface properties of lead oxide, and study the structures of the nanocomposites synthesized by a new technique of microemulsion.
Materials and Methods
Pyrrole (98%) was used as a monomer, 𝐹𝑒𝐶𝑙3 (97%) as oxidant, ethanol (99.8%) for washing, ethyl acetate (99.5%) as oil phase, 1-butano (99.9 %) as cosurfactant, Fe (NO3)3. 9H2O (98%), Pb (NO3)2 (99.2%) as iron and lead precursors respectively were purchased from Sigma Aldrich. Sodium dodecyl sulfate (SDS) (99%) was used as a surfactant obtained from Acros. NaOH (96%) was used as a precipitating agent obtained from Junsei Chemical and acetone (99.9%) was used for wash purchased from Fisher Chemical.
Techniques
XRD (PW1730 model from Philips) was used to study the structural properties; Raman (Tehran N1-541 model from Tescan) was used to investigate the vibrational characteristics; the morphologies were explored by FESEM-EDS (MIRA III model from TESCAN); VSM (VSM1100 model from Weistron) was used to examine the magnetic properties; TGA (Q600 model from TA) was used to study the thermal stability and the electrical properties of nanomaterials were investigated by CONDUCTIVITY (Pl-700PC model from Gondo).
Experimental details
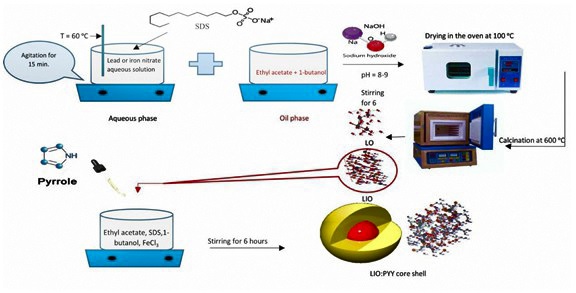
The synthesis of nanocomposites was completed according to the following synthesis steps:
Step 1: microemulsion synthesis of lead oxide (LO)
12.4204 gm of Pb (NO3)2 was dissolved in 25 gm of deionized water. 5 gm of SDS was added to the given solution and agitated for 15 minutes on a magnetic hotplate at 60 °C. This solution was added to a mixture of 14.2976 gm ethyl acetate and 5 gm of 1-butanol to yield a clear mixture and stirred for 1 hour. The precipitate formed at pH 8-9 when the above mixture was titrated with 1.5 M NaOH. The produced precipitate was agitated for 3 hours, filtered and washed multiple times with acetone and ethanol mixture (1:2), then washed with water and dried for 24 hours at 100 °C, after that calcined in the furnace for 3 hours at 600 °C.
Step 2: microemulsion synthesis of lead iron oxide (LIO)
Fe (NO3)3.9H2O (8.5 gm) and Pb (NO3)2 (5.5 gm) were dissolved in 25 grams of deionized water. 5 gm of SDS was added to the prior solution and stirred on a magnetic hotplate at 60 °C for 15 minutes. To make a clear mixture, this solution was mixed with 14.297 gm of ethyl acetate and 5 gm of 1-butanol and agitated for 1 hour. When the above mixture was titrated with 1.5 M NaOH, a precipitate occurred at pH 8-9. The precipitate was agitated for 3 hours, filtered and washed several times with a 1:1 combination of acetone and ethanol then washed with water and dried for 24 hours at 100 °C. Finally, it was calcined in the oven for 3 hours at 600 °C.
Step 3: microemulsion synthesis of lead iron oxide polypyrrole (LIOPYY)
For 3 minutes ultrasonication was employed to disperse 8.3702 grams of synthesized LIO into ethyl acetate. 5 gm SDS, 5 gm 1-butanol as a co-surfactant, 14.297 gm ethyl acetate as the oil phase and 25 mL of 1.5 M iron (III) chloride solution as the aqueous phase were used to make a microemulsion system. While vigorous stirring on a magnetic hotplate, 2.5110 grams of freshly distilled pyrrole was introduced to the system. The microemulsion was polymerized in 6 hours at room temperature with agitation. To completely remove the surfactant residue, the precipitate was rinsed with small volumes of de-ionized water then washed with acetone followed by three washes with a mixture of acetone and ethanol (1:2). The product was then dried at 60 °C for 24 hours.
Results and discussion
XRD study
The XRD patterns of the nanopowders produced are presented in Figure.1. LIO has eleven maxima at 29.9751 and d-spacing 2.98109 and LIOPYY has eighteen maxima at 30.0657 and d-spacing 2.97231. Acta Crystallographic Database Code Amcsd 0010011 was used to match the XRD peaks of lead oxide. Peaks at positions of 29.1, 30.05 and 52.93 were found to be properly indexed to the β-LO (massicot) type with an orthorhombic crystal structure [40-45]. XRD software was used to obtain Miller indices which were indicated at the XRD peaks.

Figure 1: XRD diffractograms of β-LO, LIO and LIOPYY
Some peaks in LO disappeared as 2-theta 14.57 and 2-theta 32.22 in XRD diffractograms of LIO nanoparticles, while others were created as 2-theta 27.14 and 2-theta 33.36, indicating the development of the new compound. According to Acta Crystallographic with Database code Amcsd 0017807, lead oxide was of massicot type, and the presence of peaks at 2-theta 30.05, 37.28, and 62.83 belonged to hematite (Fe2O3). The matching of XRD patterns at 2-theta 28.29, 31.41, and 54.64 in the LIOPYY nanocomposite explains that the lead oxide is of litharge type, whereas the peaks at 2-theta 30.16, 35.36, and 53.66 belong to hematite. Peaks at 2-theta 22.69, 28.40, and 44.34 show that polypyrrole was produced within the nanocomposite [46].
Scherrer's equation [85,89,91,92] was used to compute the crystalline size (D) of the LO product.
D = 0.9λ/β cos θ (1)
Where λ= 1.54056 ᵒA, β is the full breadth at half-maximum intensity (FWHM) and θ is the diffraction angle in degrees. The crystallographic parameters for nanocomposites are illustrated in Table 1.

From Table 1, it was found that the density of the unit cell decreased due to an increase in unit cell volume and a decrease in the number of the unit cell. It was noticed that an increase in the dislocation density was due to an increase in the deformations and defects in the crystal lattice due to the interactions between components [47]. LO had a higher crystallinity while LIOPYY had a lower crystallinity. This is because of the increase in amorphousness due to the amorphous Fe2O3 [94] and amorphous polypyrrole [48-50].
The structure solution process by powder diffraction data, indexing the peaks, space-group determination was determined [51]. Figure 2 illustrates the crystal structure of massicot, which has an orthorhombic crystal system.
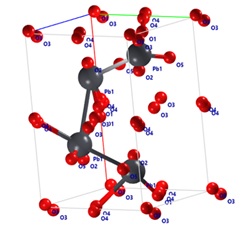
Figure 2: Crystal structure of β-LO
The types of atoms and their intensities, lengths of bonds and angles of LO, LIO and LIOPYY crystals were estimated. It was clear that the intensity of lead atoms has greater intensity while carbon has lower intensity this is related to the atomic mass of atoms.

Raman study
As shown in Figure 4, Raman spectra obtained across the spectral range (60 cm-1 – 3500 cm-1) revealed distinctive massicot peaks at (87.0160, 143.1100, 286.5247, and 382.3389) cm-1. The Raman bands accord well with the results of prior research [52]. With differences in the synthesis process and vacancy distribution in the unit cell, the relative location of the peaks can change [53]. Figure 4 shows the loss of massicot peaks (143.1100, 286.5257, and 382.3389) cm-1 and the production of additional peaks (135.3543, 267.5372, and 349.1188) cm-1, as well as weak hematite peaks (501.7257 and 604.7641) cm-1, indicating the formation of the composite (LIO).
According to Forbes Pigment Collection, Museum of Fine Arts, Boston, IRUG Filename: RMP00042, the peak at 149 cm-1 is a lead oxide of the litharge type (α-LO). Hematite is represented by the peak at 445 cm-1, while polypyrrole is represented by the peaks at (1050, 1350, and 1550) cm-1 [54]. The results obtained using Raman technique are equivalent to those obtained using the XRD technique.
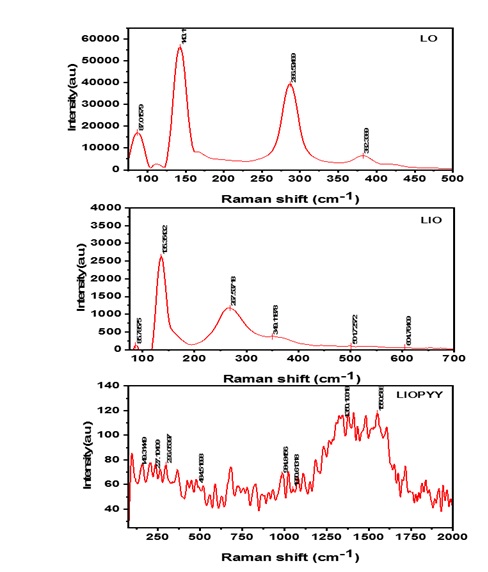
Figure 4: Raman spectra of β-LO, LIO and LIOPYY
FESEM analysis
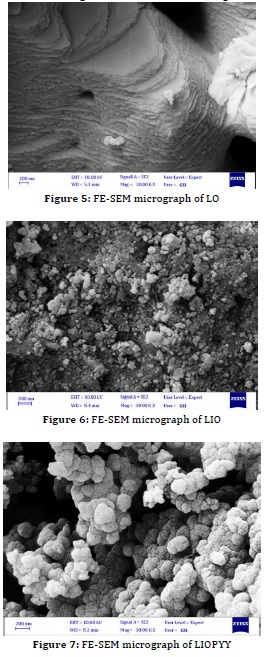
To explore the surface morphology of the prepared nanomaterials, field-emission scanning electron microscopy was used. Figure 5 depicts the morphology of LO having the nanoplates structure prepared using the microemulsion process. LIO has the shape of granulated nanoparticles, as seen in Figure 6. Because of various types of attractive forces, such as hydrogen bonds, Vander Waal forces, Coulomb forces, and physical friction between the fine nanoparticles, the scanning images show a semi-uniform distribution of particle size and the particles appear to be spherical and agglomerated. LIOPYY nanocomposites had a homogeneous globular morphology, although the hemispheric nature of the polypyrrole particles clustered, as illustrated in Figure 7.
VSM analysis
At room temperature, magnetization measurements of nanocomposites were carried out utilizing the vibrating sample magnetometry analysis (VSM) technique using hysteresis curves of the samples at fields ranging from -10000 to 10000. Figure 8 depicts a hysteresis loop that demonstrates a linear increase in magnetization with an applied magnetic field, confirming the paramagnetic behavior of LO. In addition, Figure 8 depicts the existence of ferromagnetic material (-Fe2O3) as a result of increased magnetism as compared with LO. Two sublattices exist in magnetic materials, each with a different magnetic moment. As a result, even if the moments have the same sign, they do not cancel, resulting in a nonzero net moment. The ferrimagnetic state is a state that exists in between ferro-and antiferromagnetic states. As a result, in terms of magnetic properties, a ferrimagnet is comparable to a ferromagnet at any given temperature.
Magnetite (Fe3O4), is an excellent example of a ferromagnetic material. The Fe2 + and Fe3+ sites have opposite and unequal moments. Maghemite (γ-Fe2O3) is a well-known ferrimagnetic oxide material with an intermediate iron-oxidation state that is frequently employed in the magnetic recording industry [55]. The Fe2O3 core of LIOPYY core shells gives them their magnetic properties. In addition, the polypyrrole shell presence on the Fe2O3 core surface has been represented by a small amount in the Ms value for LIOPYY core shells [56,57].
From Table 11, it is found that β-LO has a lower saturation magnetization (Ms) of 0.03360 emu/g while LIO core-shell has a higher Ms of 16.4069 emu/g. This might be due to the maghemite shell that surrounds the nanoparticles, formed by oxidizing α-Fe2O3 to ɣ-Fe2O3. Ferric oxide nanoparticles showed superparamagnetic properties with a small hysteresis loop, meaning that in the absence of an external field, thermal energy may overcome a single particle's anisotropic energy barrier, and the particles' net magnetization is zero [58].

Conductivity measurements
The AC electrical conductivity and dielectric characteristics of all produced samples were investigated. For AC conductivity and dielectric tests, a frequency range of 50 Hz to 1 MHz was chosen. In the low-frequency range, β-LO nanoparticles do not display ac conductivity, and this trend persists up to 40 kHz, whereas LIO and LIOPYY nanoparticles trend up to 3.5 kHz and 2.5 kHz, respectively. As seen in Table 12, LIOPYY has a greater conductivity of 0.3219 Sm-1, while LO has a poorer conductivity of 7.1437 x 10-8 Sm-1.
The conductivity of synthesized nanocomposites rises with frequency, notably at high frequencies, as compared with -LO alone, due to the creation of large charge carriers of Fe2O3 and polypyrrole as a result of its own adequate energy. It is a non-continuous leap transition charge carrier or their reorientation between local levels of crystal boundaries that enhances the frequency of the electric field applied to the samples [59].
As shown in Figure 10, the relationship between real dielectric constant and frequency was explored. The real dielectric constant reduces substantially from 50 Hz to 1 Mz, as seen in table 12 and Figure 10. The real dielectric constant fell at 1 MHz when compared with 50 Hz. Under the influence of an alternating electrical field, the imaginary dielectric constant indicates energy absorption and dispersion at intervals (local defects, grain boundaries, and the accumulation of local charges). Because of the mobility of charge carriers and their high numbers across matter, as shown in Figure 11, the imaginary dielectric constant increases with decreasing frequency, making it possible to restrict their mobility in the case of phase interaction.
The other explanation is phase multiplicity, which results in a reduction in scattered energy. With an increase in frequency, all of these factors lead to a decrease in the imaginary dielectric constant. The loss coefficient (tan) as a function of frequency is shown in Figure 12. For all samples, it was discovered that the loss coefficient (tan) decreases with increasing frequency. The dielectric loss is caused by thermal irritation and friction, which obstructs the dipoles' alignment and rotation in the field.

Thermogravimetric analysis
The thermal stability and thermodynamic parameters of prepared nanocomposites were studied by thermogravimetric analysis. The setup began with initial weights of 6.823 mg, 3.540 mg, and 4.308 mg for LO, LIO, and LIOPYY, respectively and the range of heating was (40-800) ⁰C using inert gas argon with a flow rate of 40 mL/min. The thermal behavior of the nanocomposites was indicated by the TGA plot between weight loss and temperatures, as shown in Figure 13. It was found that LO and LIO nanocomposites were very stable and did not undergo any significant decomposition in the temperature range, while LIOPYY showed relative thermal stability. As shown in Table 13, the weight loss of LO, LIO, and LIOPYY at 40–460 ⁰C was 0.582, 1.159, and 0.928% with onset temperatures of 239.78, 160.3, and 161.97, respectively. This stage is associated with the loss of a very small percent of humidity, in which LIO loses more than others due to its large pore diameter in comparison with LO, having low porosity due to its small diameter.
The second stage was (420-800) ⁰C, which corresponds to the liberation of adsorbed oxygen molecules. At this stage, LO, LIO, and LIOPYY had a weight loss percent of (0.4506, 1.731, and 1.429) ⁰C corresponding to onset temperatures of 777.08, 629.41, and 662.82, respectively. In both temperature ranges, LIO liberates a greater amount of humidity than other nanoparticles in both temperature ranges. The thermogravimetric functions of LO, LIO, and LIOPYY were illustrated in Tables 14, 15, and 16, respectively for the selected temperature ranges (150-400) ⁰C and (420-630) ⁰C. It was found that the activation energy (ΔEa) had the maximum value for LO while LIOPYY had the minimum value. At (150-400) ⁰C for, the process was endothermic, while the range (420-630) ⁰C was exothermic. The change in entropy (ΔS) was increased by adding more components to LO (iron oxide and polypyrrole) because of the increase in the porosity and amorphous nature, and the change in the free energy was a minus sign, indicating the spontaneous nature of the processes.


Conclusion
Lead oxide and its new nanocomposites were synthesized using a new microemulsion process. The lead oxide was of the massicot type, while the iron oxide was of the hematite type according to the XRD examination. The fact that the surface morphology of nanocomposites changed significantly suggested that the synthesis had been effective. Nanocomposites showed a shift in wavelength according to Raman spectra and the results were equivalent to XRD analyses. With increasing frequency, conductivity increased and the LIOPYY had a higher conductivity. VSM study revealed that LIO had a high magnetism due to the presence of more magnetic hematite and TGA examination showed that the nanocomposites were stable.
Funding
This research did not receive any specific grant from fundig agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID
Hadi Beitollai
https://www.orcid.org/0000-0002-0669-5216
HOW TO CITE THIS ARTICLE
Ahmed K. Abass, Abdul Karim M. A. Al-Sammarraie, Synthesis of New PbO-Fe2O3-Polypyrrole Hybrid Nanocomposite to Improve the Structural, Magnetic and Electrical Characteristics of Lead Oxide. Chem. Methodol., 2022, 6(4) 301-318
- Sanchez C., Julián B., Belleville P., Popall M., Mater. Chem., 2005, 15:3559 [Crossref], [Google Scholar], [Publisher]
- Faustini M., Nicole L., Ruiz-Hitzky E., Sanchez C., Funct. Mater., 2018, 28:1704158 [Crossref], [Google Scholar], [Publisher]
- Judeinstein P., Sanchez C., Mater. Chem., 1996, 6:511 [Crossref], [Google Scholar], [Publisher]
- Rebber M., Willa C., Koziej D., Nanoscale Horiz., 2020, 5:431 [Crossref], [Google Scholar], [Publisher]
- Zhang X., Chen Z., Mou K., Jiao M., Zhang X., Liu L., Nanoscale, 2019, 11:18715 [Crossref], [Google Scholar], [Publisher]
- Jiang Z., Wang Y., Zhang X., Zheng H., Wang X., Liang Y., Nano Res., 2019, 12:2330 [Crossref], [Google Scholar], [Publisher]
- Khan M., Khurram A.A., Tiehu L., Zhao T.K., Xiong C., Ali Z., … Ullah A., Relat. Mater., 2017, 78:58 [Crossref], [Google Scholar], [Publisher]
[8]. Narayan N., Meiyazhagan A., Vajtai R., Materials, 2019, 12:3602 [Crossref], [Google Scholar], [Publisher]
- Vega-Jiménez A.L., Vázquez-Olmos A.R., Acosta-Gío E., Álvarez-Pérez M.A., In Nanoemulsions-properties, fabrications and applications, 2019 [Crossref], [Google Scholar], [Publisher]
[10]. Rehman A.U., Khan M., Maosheng Z., J. Energy Storage, 2019, 26:101026 [Crossref], [Google Scholar], [Publisher]
- Hayat A., Raziq F., Khan M., Khan J., Mane S.K.B., Ahmad A., Khan W.U., Colloid Interface Sci., 2019, 554:627 [Crossref], [Google Scholar], [Publisher]
- Bigdeli A., Ghasemi F., Golmohammadi H., Abbasi-Moayed S., Nejad M.A.F., Fahimi-Kashani N., Hormozi-Nezhad M.R., Nanoscale, 2017, 9:16546 [Crossref], [Google Scholar], [Publisher]
- Stäb J., Furin D., Fechner P., Proll G., Soriano-Dotor L.M., Ruiz-Palomero C., Gauglitz G., Optical Sensors 2017, 2017, 102310O [Crossref], [Google Scholar], [Publisher]
- Hayat A., Shaishta N., Mane S.K.B., Khan J., Hayat A., ACS Appl. Mater. Interfaces, 2019, 11:46756 [Crossref], [Google Scholar], [Publisher]
- Ahmed A., Baig H., Sundaram S., Mallick T.K., J. Photoenergy, 2019, 2019:1 [Crossref], [Google Scholar], [Publisher]
[16]. Madkour L.H., Nanoelectron. Mater., 2019, 565–603 [Crossref], [Google Scholar], [Publisher] [17]. Qu Z., Wang L., Tang H., Ye H., Li M., Nanomaterials, 2019, 9:956 [Crossref], [Google Scholar], [Publisher]
- Güler O., Erdemir F., Çelebi M., Çuvalcı H., Çanakçı A., Results Phys., 2019, 15:102700 [Crossref], [Google Scholar], [Publisher]
[19]. Rehman A.U., Hayat A., Munis A., Zhao T., Israr M., Zheng M., Proc. Inst. Civ. Eng. Energy, 2020, 173:60 [Crossref], [Google Scholar], [Publisher]
- Yunus I.S., Harwin Kurniawan A., Adityawarman D., Indarto A., Technol. Rev., 2012, 1:136 [Crossref], [Google Scholar], [Publisher]
- Parveen H., Mukhtar S., El Sayed N.H., Hayat F., Asian J. Chem., 2014, 26:8134 [Crossref], [Google Scholar], [Publisher]
- Hayat A., Raziq F., Khan M., Ullah I., Rahman M.U., Khan W.U., Ahmad A., Photochem. Photobiol. A: Chem., 2019, 379:88 [Crossref], [Google Scholar], [Publisher]
- Miri A., Sarani M., Hashemzadeh A., Mardani Z., Darroudi M., Green Chem. Lett. Rev., 2018, 11:567 [Crossref], [Google Scholar], [Publisher]
[24]. Jiao Y., Liu Y., Qu F., Wu X., CrystEngComm., 2014, 16:575 [Crossref], [Google Scholar], [Publisher] [25]. Jiao Y., Liu Y., Qu F., Umar A., Wu X., J. Colloid Interface Sci., 2015, 451:93 [Crossref], [Google Scholar], [Publisher]
- Zheng X., Jiao Y., Chai F., Qu F., Umar A., Wu X., Colloid Interface Sci., 2015, 457:345 [Crossref], [Google Scholar], [Publisher]
[27]. Peng D., Beysen S., Li Q., Sun Y., Yang L., Particuology, 2010, 8:386 [Crossref], [Google Scholar], [Publisher] [28]. Dai M., Zhao L., Gao H., Sun P., Liu F., Zhang S., Lu G., ACS Appl. Mater. Interfaces, 2017, 9:8919 [Crossref], [Google Scholar], [Publisher]
- Gupta K., Jana P.C., Meikap A.K., Met., 2010, 160:1566 [Crossref], [Google Scholar], [Publisher]
[30]. Ko S.H., Park I., Pan H., Grigoropoulos C.P., Pisano A.P., Luscombe C.K., Fréchet J.M.J., Nano Lett., 2007, 7:1869 [Crossref], [Google Scholar], [Publisher] [31]. Tabchouche A., Ourari A., Zoubeidi N., zerrouki D., Energy Procedia, 2013, 36:1009 [Crossref], [Google Scholar], [Publisher]
- Arbain R., Othman M., Palaniandy S., Eng., 2011, 24:1 [Crossref], [Google Scholar], [Publisher]
[33]. Song K.C., Lee S.M., Park T.S., Lee B.S., Korean J. Chem. Eng., 2009, 26:153 [Crossref], [Google Scholar], [Publisher]
- Ghosh S.K., Kundu S., Pal T., Mater. Sci., 2002, 25:581 [Crossref], [Google Scholar], [Publisher]
[35]. Mohanty U.S., J. Appl. Electrochem., 2010, 41:257 [Crossref], [Google Scholar], [Publisher] [36]. Hayashi H., Hakuta Y., Materials, 2010, 3:3794 [Crossref], [Google Scholar], [Publisher] [37]. Pérez-Tijerina E., Gracia Pinilla M., Mejía-Rosales S., Ortiz-Méndez U., Torres A., José-Yacamán M., Faraday Discuss., 2008, 138:353 [Crossref], [Google Scholar], [Publisher] [38]. Sonawane R.S., Dongare M.K., J. Mol. Catal. A: Chem., 2006, 243:68 [Crossref], [Google Scholar], [Publisher]
- López-Quintela M.A., Opin. Colloid Interface Sci., 2003, 8:137 [Crossref], [Google Scholar], [Publisher]
[40]. Karami H., Karimi M.A., Haghdar S., Mater. Res. Bull., 2008, 43:3054 [Crossref], [Google Scholar], [Publisher] [41]. Kashani-Motlagh M.M., Mahmoudabad M.K., J. Sol-Gel Sci. Technol., 2011, 59:106 [Crossref], [Google Scholar], [Publisher] [42]. Wilkinson T.J., Perry D.L., Spiller E., Berdahl P., Derenzo S.E., Weber M.J., MRS Proceedings 2001, 704 [Crossref], [Google Scholar], [Publisher] [43]. Hashemi L., Morsali A., J. Inorg. Organomet. Polym. Mater., 2010, 20:856 [Crossref], [Google Scholar], [Publisher] [44]. Safarifard V., Morsali A., Inorganica Chim. Acta, 2013, 398:151 [Crossref], [Google Scholar], [Publisher] [45]. Lian J., Zhang X, Zhang H. Jiang Z. Zhang J., Mater. Lett., 2004, 58:1183 [Crossref], [Google Scholar], [Publisher]
- Feng W., Sun E., Fujii A., Wu H., Niihara K., Yoshino K., Chem. Soc. Japan, 2000, 73:2627 [Crossref], [Google Scholar], [Publisher]
[47]. Robin A., Martinez G.A.S., Suzuki P.A., Mater. Des., 2012, 34:319 [Crossref], [Google Scholar], [Publisher] [48]. Jang, J., Sim K., Polymer, 1997, 38:4043 [Crossref], [Google Scholar], [Publisher] [49]. Jang J., Won J., Polymer, 1998, 39:4335 [Crossref], [Google Scholar], [Publisher] [50]. Bae W.J., Jo W.H., Park Y.H., Macromol. Res., 2002, 10:145 [Crossref], [Google Scholar], [Publisher] [51]. Altomare A., Cuocci C., Giacovazzo C., Moliterni A., Rizzi R., Corriero N., Falcicchio A., Journal of Applied Crystallography, 2013, 46:1231 [Crossref], [Google Scholar], [Publisher]
- Burgio L., Clark R.J., Firth S., Analyst, 2001, 126:222 [Crossref], [Google Scholar], [Publisher]
- Faria, D.L.D., Venâncio Silva S., De Oliveira M.T., Raman spectrosc., 1997, 28:873 [Crossref], [Google Scholar], [Publisher]
[54]. Choi Y.M., Lim H., Lee H.N., Park Y.M., Park J.S., Kim H.J., Biosensors, 2020, 10:111 [Crossref], [Google Scholar], [Publisher] [55]. Mohamed A.M.O., Paleologos E.K., Fundamentals of Geoenvironmental Engineering, 2018, 3–42 [Crossref], [Google Scholar], [Publisher][56]. Song X., Gong H., Yin S., Cheng L., Wang C., Li Z., Liu Z., Adv. Funct. Mater., 2013, 24:1194 [Crossref], [Google Scholar], [Publisher][57]. Vickers N.J., Curr. Biol., 2017, 27:R713 [Crossref], [Google Scholar], [Publisher] [58]. Qi H. Yan B., Li C. Lu W., Mech. Astron., 2011, 54:1239 [Crossref], [Google Scholar], [Publisher]
[58]. Rafic S.N., Al-Jawad S.M.H., Muhsen M.M., Al-Nahrain Univ. Sci., 2017, 20:91 [Crossref], [Google Scholar], [Publisher]