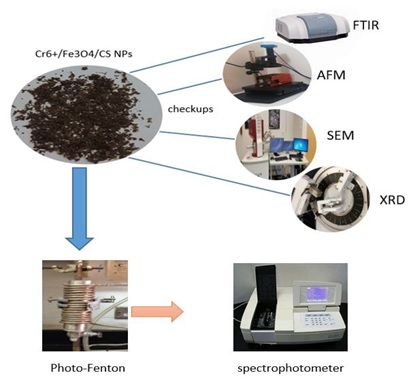
Document Type : Original Article
Authors
Department of Chemistry, College of Science, Baghdad University, Iraq
Abstract
Removing heavy metal ions from organic contaminants by industrial wastewater treatment systems is a difficult challenge. In this study, organic contaminants were degraded using functional composite hydrogels with photo-Fenton reaction activity. Chromium, chitosan, Fe3O4, and other ingredients go into the making of the hydrogel. Following are some factors that changed photo-Fenton activity: (pH, H2O2 conc., temp, and exposure period). AFM was used to analyze the composite's morphology and typical diameter (AFM). It investigated how much chromium adsorbs on chitosan (BET). After 60 minutes of UV exposure, The MB dye degradation in the Cr6+/Fe3O4/CS hydrogel composite was 90.4%.
Graphical Abstract
Keywords
Main Subjects
Introduction
The traditional Fenton reaction, used to treat wastewater, is an example of an advanced oxidation technology. In this reaction, ferrous iron (Fe2+) and hydrogen peroxide (H2O2) are combined to produce hydroxyl radicals (OH) [1]. Homogeneous Fenton reactions have a number of drawbacks, including an acidic pH, efficient H2O2 consumption, and the production of sludge [2]. These drawbacks limit their potential applications. A big H2O2-Fe2+ molar ratio and the requirement for a lot of Fe2+ further raise the requirement for reagents [3]. For the degradation of organic pollutants, the heterogeneous Fenton reaction employs solid, iron-containing compounds or solid materials rich in iron, including clay [4], mesoporous silica [5], and activated carbon [6], supported iron-containing compounds, Fe3O4 [7, 8], Fe2O3 [9]. Comparatively, the heterogeneous Fenton reactions are a successful oxidative method used to destroy organic contaminants that can effectively enhance H2O2 conversion with little breakdown, the preparation of hydrogels with exceptional mechanical characteristics was motivated by high attendance [10]. In order to make a hydrogel with high strength, there are typically three different recipes: a topological gel [11], a double network gel [12], and a composite gel [13]. Composite gels are among these recipes and are thought to improve the mechanical properties of hydrogels; for example, composite gels with a special organic-inorganic network structure would have extraordinary mechanical properties [14]. UV addition to the Fenton process may be a crucial ally in the decolorization of dye due to its capacity to impact the direct production of OH radicals. The photo Fenton procedure, which involves exposing Fenton to UV light, may accelerate the degradation of organic pollutants. By reducing Fe3+, UV light also encourages the recycling of ferrous catalyst and increases the formation of hydroxyl radicals. By using this method, Fe2+ concentrations will increase, and the reaction as a whole will accelerate. AOPs' dye degradation and decolorization have been successfully treated with oxidation using photo-Fenton and Fenton reagents [15]. Fenton's reagent, based on ferrous ion and hydrogen peroxide, is used to oxidize material by taking advantage of the hydroxyl radicals produced in an acidic solution as a result of the catalytic breakdown of H2O2 [16]. Chitosan is a water-soluble polymer and is widely used in industrial water treatment. It is treated as non-toxic, economical, and efficient and undoubtedly has great potential application prospects. CS is a low-cost bio-adsorbent and disinfectant with a number of advantages, including the amine functional group, which is totally reactive among metal ions, biocompatibility, biodegradability, and the safety of their use due to their non-toxic behavior [17]. The hydrogels Cr6+/Fe3O4/CS is efficient to adsorb heavy metals this is because of the chemical adsorption ascribed to the metal NH2 complex.
Materials and Methods
Determination of maximum absorption (λmax)
The amount of MB absorption was calculated using the wavelength value of 661 nm, as shown in Figure 1. MB dye was chosen as a measure to study the surface area to know the rate of degradation through photo Fenton reaction and consider the dye as a pollutant instead of polluted water. An ultraviolet visible absorption spectrum was performed for MB blue. The absorption spectrum is shown as peaks between absorbance and wavelength.
Figure 2 illustrates the calibration curve used to determine a linear equation using Beer-law Lambert's to determine a substance's concentration and the relationship between concentration and absorbance.
Synthesis of CS/Fe3O4 nanocomposite
Chitosan in 1% acetic acid solution and stirring was dissolved continuously for 10 minutes to create well-oxidized nanocomposites via the Fenton reaction.
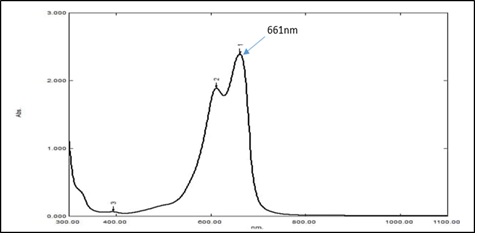
Figure 1: The absorption spectrum for M.B. dye by UV-Vis

Figure 2: Calibration curve of MB
Iron oxide was added once the suspension was well-suspended. The solution was then thoroughly mixed up in an ultrasonic device for 4 minutes to ensure no agglomeration of the particles. This is carried out at 20 °C. After that, we gradually add the ammonium hydroxide solution and put the mixture back into the ultrasonic device to create a homogeneous solution of the magnetic compound. We then allow it to dry for 24 hours, as indicated in Figure 3, a magnetic crystalline powder is obtained, and this powder is dissolved and added to a 20-ppm chromium solution before the mixture is added to the Fenton cell [18].
Figure 4 and 5 show the interaction of chromium and iron oxide with chitosan through Fourier Transform Infrared spectroscopy (FT-IR) due to the shift of all peaks and the appearance of new peaks between 400-800 cm-1, indicating the interaction of one of the transition elements, which is chrome with the original compound.
Preparation of photocell
A stainless-steel tube with a diameter of 4 cm and a length of 15 cm was outfitted with a copper coil around the outer cell surface and connected to a water bath to control the reactor heat and lamp. First, the inner cell surface is treated with concentrated HF acid, producing the inside of the cell or photo that is also rough and could take up paint. After 10 minutes, the Fenton-filled reactor Cr/Fe3O4/CS compound suspension is decanted to allow a stable coating layer to form [19]. For the purpose of impregnating the Cr/Fe3O4/CS catalyst photo reactor layer. In order to increase the efficiency of the cell's ability to oxidize well, it is exposed to 500 °C until the inner surface is compressed with the compound. Repeat these steps several times to make the coating more than one layer, and the presence of catalysts for the reaction, such as hydrogen peroxide, is added to the dye to test the coating's stability. Figure 6 displayed the equipment for the degrading process and the suspension composite covering the cell.

Figure 3: Cr6+/Fe3O4 /CS composite
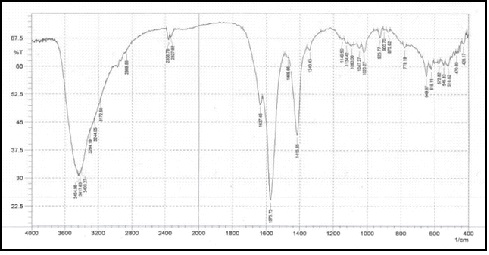
Figure 4: The FT-IR spectrum of chitosan

Figure 5: The FT-IR spectrum of Cr6+/ Fe3O4 /CS composite

Figure 6: Shows how to set up the entire system for photo degradation
Results and Discussion
Atomic force microscope
According to the AFM investigation, the composite of Cr6+, Fe3O4, and CS has measurements for its average grain size and granularity cumulating distribution. The parameters average roughness (Sa) and square roughness are the most often utilized (Sq). The data of Cr6+/Fe3O4/CS were (roughness average=7.400, root mean square=8.496, surface skewness=-0.2388, average diameter=12.66) nm. As shown in Figure 7.
Brunauer–emmett–teller (BET)
A theoretically valid isotherm equation for the equilibrium of gas-solid systems is Brunauer-Emmett-Teller. The multilayer adsorption theory (BET), developed as an extension of the Langmuir technique, stipulates that there is a dynamic equilibrium between adsorbate molecules in succeeding layers, and adsorbed molecules may condense on it after the development of a molecular monolayer [20]. They provide the equation commonly referred to as the BET (Equation 1), which:
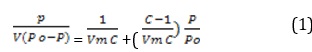
The BET specific surface area (as) calculated from the BET plots for the adsorption of Cr6+ ion onto CS or CS/Fe3O4. The aS, BET increased from 2.7269 m2 g-1 for CS to 6.5481 m2 g-1 for CS/ Fe3O4 and 12.5032 m2 g-1 for Cr6+/Fe3O4/CS indicates that when fe3o4 is added to CS, the surface area of CS/ Fe3O4 increases, when Cr6+ ion was added to CS/ Fe3O4 composite, the surface area was significantly increased as shown in Figure 8 and 9.
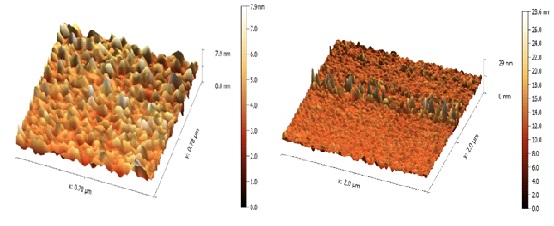
Figure 7: AFM images for Cr6+/Fe3O4/CS
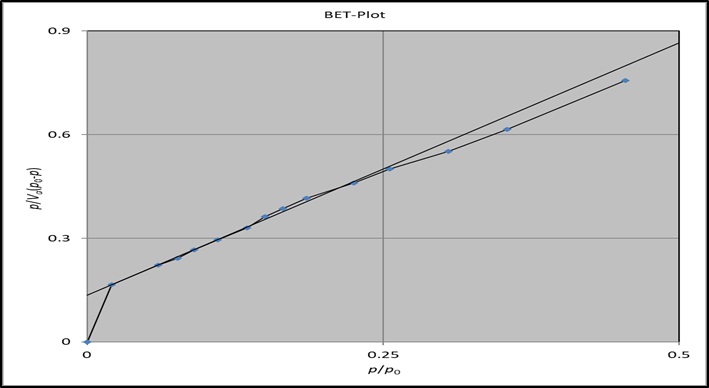
Figure 8: BET plots of CS adsorption isotherms

Figure 9: BET plots of Cr6+/Fe3O4/CS adsorption isotherms
Effect of H2O2 concentration
The concentration of hydrogen peroxide is one of the operational parameters that significantly influences the ultimate mineralization extent. Degradation efficiency also rises with further increases in the optimal Fenton reagent ratio when a particular point is achieved (H2O2 conc.). At 298 K, pH=7 after 60 min in the presence of catalyst CS, Cr6+/Fe3O4/CS composite, the impact of H2O2 conc. on the MB degradation was investigated, and the rise in % deg. [21] with H2O2 conc. increased, as shown in Figure 10.
Effect of Cr6+ ion concentration
After 60 minutes, in the presence of CS and Cr6+/Fe3O4/CS, hydrogen peroxide (5×10-3M), the chromium ion concentration affected the percentage of MB at 7 ppm. In order to maintain stable levels of the dye solutions in the compounds, the photolysis method was carried out at pH 7 and 298 K. The location where the dye-pollutant mixture and chromium solution were combined. The degradation rate was greater than that of chitosan with the combination Cr6+/Fe3O4/CS. Figure 11 illustrates that 20 ppm of chromium was the ideal amount to employ in this operation.
Effect of temperature
The effects of temperature on the degradation of MB dye have been studied using Cr6+/Fe3O4/CS composite, MB dye (7 ppm), H2O2 concentration (5×10-3) M, pH=7, and temperatures of (293, 303, 313 and 323) K. Following then, samples were taken every 5 minutes; after UV exposure. Figure 12 demonstrates the determination of %deg.
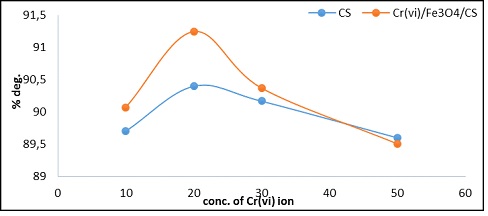
Figure 10: Shows the catalyst effect of varied H2O2 conc. for (7 ppm M.B) after 60 min at 298K, pH=7
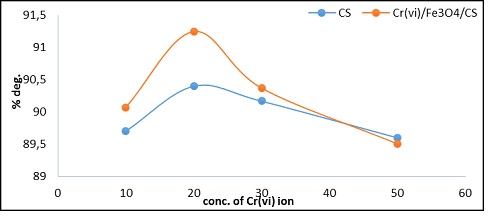
Figure 11: Shows the impact of changing the Cr6+ ion concentration for M.B. (7ppm) concentration, 5×10-3 (H2O2 conc.) after (60 min.), at 298K, pH=7 on the CS, Cr/Fe3O4/CS compound catalyst

Figure 12: Shows how the temperature-dependent variation of 7 ppm M.B with (5×10-3 H2O2 conc.), %deg. by Cr6+/Fe3O4/cs composite
Kinetic degradation study
The first order was applied to the deteriorating reaction of 7 ppm M.B and 5×10-3 M of H2O2 using a CS/Fe3O4 composite. At a pH of 7, the connection between temperature and degradation rate was examined. It could be fitted into relation curves, showing the first-order kinetics of the reaction as given in the following (Equation 2) [22]. Figure 13 displays kinematic calculations by Ln Ct and time.
![]()
Where: C0: initial concentration of M.B, Ct: concentration of M.B after exposing to UV at time, K: rate constant and t: time.
The anti-arrhenius equation was applied to determine the kinetic parameter A, Ea, as shown in Figure 14: (Equations 3 and 4) [23].
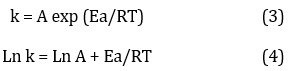
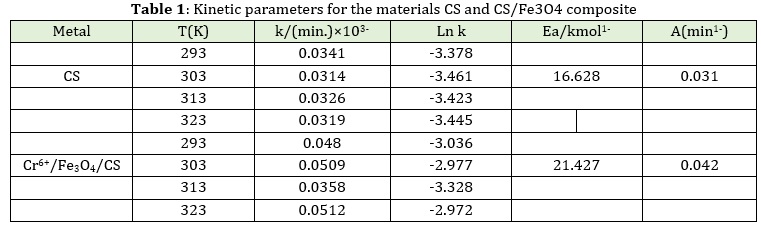
Where T: is the absolute temperature (measured in kelvins), k is the rate constant, Ea is the reaction's activation energy (measured in kilojoules per mole), A is the pre-exponential factor, and R is the universal gas constant. In the rate equation, Ea stands for the activation of degradation (the least amount of energy needed to initiate a chemical process). A stands for the pre-exponential component. The values of Ea and A are then determined by the slope and intercept of the Ln k against the 1/T plot, respectively, and are shown in Table 1.

Figure 13: linear relations between Ln Ce and time for (7 ppm M.B deg.) by a) CS, B) Cr6+/Fe3O4/CS composite at different temperatures

Figure 14: Anti-arrhenius plots, relation Lnk with 1/T for the7 ppm M.B %deg. CS coating, Cr/Fe3O4/CS composite
Conclusion
The absorption spectrum and calibration curve of methyl blue were performed as illustrated in Figure 1 and 2. The degradability of methylene blue dye on composites made of Cr, Fe3O4, and CS was investigated. By manufacturing Cr6+/ Fe3O4/CS nanocomposite as shown in the Figure 3 on the research method [24] to break down the pollutants dye (Methyl blue). The composite's characteristics, such as its typical diameter and shape, were determined using AFM. Additionally, BET was used to determine the Fe3O4 composite's surface area for chromium ion adsorption. When Cr ions are combined with CS/Fe3O4, which was diagnosed by FT-IR as in the given Figure 5, the particle size increases, and MB (% deg.) efficiency rises. The photo-Fenton approach successfully removed the contaminating dye using a Cr6+/ Fe3O4/CS composite as a catalyst. The ideal exposure period was found to be 60 minutes. The pH influence showed that MB dye on Cr/ Fe3O4/CS composite degraded fastest at pH=7. The color has been significantly reduced after the dye solution has been exposed to irradiation for a longer period of time, and the dye has been converted into organic material (COD test after 2h is low or under range). The kinetic research data support the first-order interpretation of MB dye degradation on Cr/ Fe3O4/CS composite.
Acknowledgements
Department of Chemistry, College of science, university of Baghdad and Prof. Dr. Khulood A. Saleh.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID:
Yaman Khalid Sadiq
https://orcid.org/0000-0002-1651-786X
HOW TO CITE THIS ARTICLE
Yaman Khalid Sadiq, Khulood A. Saleh. Synthesis and Characterization of Chrome (VI) ion/iron oxide/chitosan Composite for Oxidation of methylene blue by Photo-Fenton Reaction. Chem. Methodol., 2023, 7(2) 112-122
http://dx.doi.org/10.22034/CHEMM.2022.361921.1608
URL: http://www.chemmethod.com/article_159506.html