Document Type : Original Article
Authors
University of Technology (UOT), Department of Applied Science, Chemistry Division, Baghdad, Iraq
Abstract
Substituted pyridine derivatives have been synthesized by reacting 3-acetyl-2H-chromen-2-one with suitable aldehydes using ethyl acetoacetate and ammonium acetate to afford 3-acetyl-4-( substituted -phenyl)-6-(2-oxo-2H-chromen-3-yl) pyridin-2(1H)-one (2a-c). TLC has checked the homogeneity of all three synthesized compounds. Their IR, and 1H-NMR, which can reduce mild steel corrosion in 0.5 M hydrochloric acids were investigated using weight loss measurements and surface detection techniques, with scanning electron microscope (SEM) investigations utilized to identify the used surface technique. The inhibition efficiency was more significant than 99.62%. The formation of protective adsorption layers on the steel surface was credited with excellent inhibitor results. To better understand the inhibition process, quantum chemical calculations were used.
Graphical Abstract
Keywords
Main Subjects
Introduction
Numerous casualties are brought by corrosion processes, primarily in the industrial sector. It is evident that preventing it from happening in the first place is the only way to combat it. Corrosion inhibitors are among the most well-known and practical items on the market when it comes to avoid or prevent the deterioration or degradation of metal surfaces. This method is a close second to stand up due to its inexpensive cost and simplicity of use [1, 2]. Due to its high mechanical strength, ease of manufacture, and low cost, iron, and its alloys are widely used as a building material in various industrial applications, including petroleum, power plants, and chemical industries [3]. Steel comes into contact with corrosive conditions in a number of ways, including acidic solutions etching, acid pickling, etc. acid descaling, acid washing, and acidification of oil wells [4]. The chemical inhibitors use in liquid form or vapor form or both. There are two steps including inhibitor action. Transporting the inhibitor to the metal surface is the first step and the second step involves the interaction of the inhibitor and metal surface [5]. Because it contains heteroatoms like sulfur, nitrogen, and oxygen in addition to aromatic rings, an organic inhibitor performs well among other corrosion inhibitors [6]. While sulfur-containing compounds are efficient inhibitors in sulfuric acid, metals in hydrochloric acid can be effectively protected from corrosion by organic inhibitors. Nitrogen and sulfur-containing chemicals are excellent corrosion inhibitors in both mediums [7]. Inhibitors made up of organic compounds with multiple bonds are powerful. Adsorption onto the metal surface, which is normally oxide free in acid solutions, is commonly accepted to be the primary step in the action of inhibitors in acid solutions. The most widely utilized heterocyclic compounds contain the heteroatoms of sulfur (S), phosphorus (P), nitrogen (N), or oxygen (O), which act as adsorption sites. As a result of this research, three heterocyclic compounds were produced and chosen in a hydrochloric acid solution as corrosion inhibitors for steel.
Materials and Methods
Gallen Kamp (MFB-600) melting point apparatus was used to calculate melting points of compounds. Compound FT-IR spectra were observed. FT-IR 8400S spectrophotometer was provided by SHIMADZU. Bruker 400 MHz spectrophotometer, deuterated DMSO as the solvent, and TMS as an internal normal were used to obtain the 1H-NMR spectra. On silica gel TLC plates, the compounds were checked for purity, and the spots were seen using iodine vapor.
Synthesis of inhibitors
The following is a general procedure for synthesizing 3-acetyl-4-(substituted -phenyl)-6-(2-oxo-2H-chromen-3-yl) pyridin-2(1H)-one (2a-c)
The scheme of the inhibitor synthesis process is displayed in Scheme 1 (Pyridine derivatives). 3-acetyl-2H-chromen-2-one (0.005 mol), suitable aldehydes (0.005 mol), ethyl acetoacetate (0.65 g., 0.005 moles), and ammonium acetate (3 g, 0.04 mol) were used to make the compounds, which were refluxed for 24 hours [8, 9]. The substance was washed with water and collected by filtration after cooling. To be sure the reaction had finished and the chemical was pure, TLC was utilized (mobile phase hexane: methyl acetate 7:3). To obtain the title compounds, the substance was recrystallized in the required solvent.
Synthesis of 3-acetyl-6-(2-oxo-2H-chromen-3-yl)-4-phenylpyridin-2(1H)-one (2a)
Light yellow solid, yield 59%, mp 296-298 °C, Rf= 0.55, IR (KBr) (νmax/ cm-1): 3115 (NH), 1722, 1674, and 1656 (3C=O) cm-1; 1H-NMR (DMSO-d6, 400 MHz): δ 2.3 (s, 3H, CH3), 7.1-7.9 (m, 11H, Ar-H and C=CH), and 10.5 (s, 1H, NH).
Synthesis of 3-acetyl-4-(4-nitrophenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2(1H)-one (2b)
Yellow solid, yield 62%, mp 276-278 °C, Rf= 0.57, IR (KBr) (νmax/ cm-1): 3188 (NH), 1718, 1676, 1627 (3C=O), 1514, 1346 (p-NO2), 1H-NMR (DMSO-d6, 400 MHz): δ 2.99 (s, 3H, CH3), 7.39–8.95 (m, 10H, Ar-H and C=CH), ), and 11.16 (s, 1H, NH).
Synthesis of 3-acetyl-4-(4-hydroxy-2-methoxyphenyl)-6-(2-oxo-2H-chromen-3-yl)pyridin-2(1H)-one (2c)
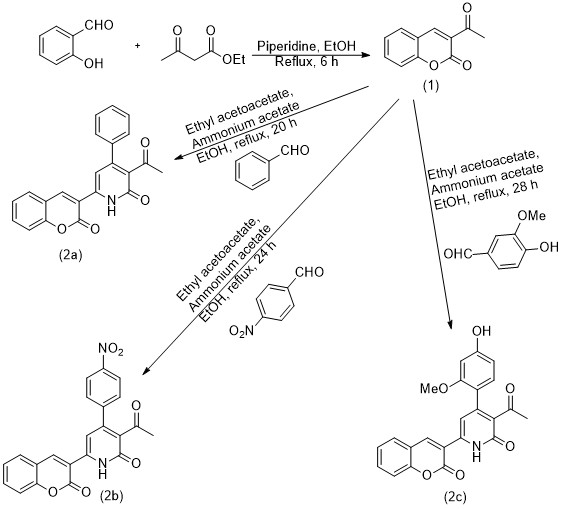
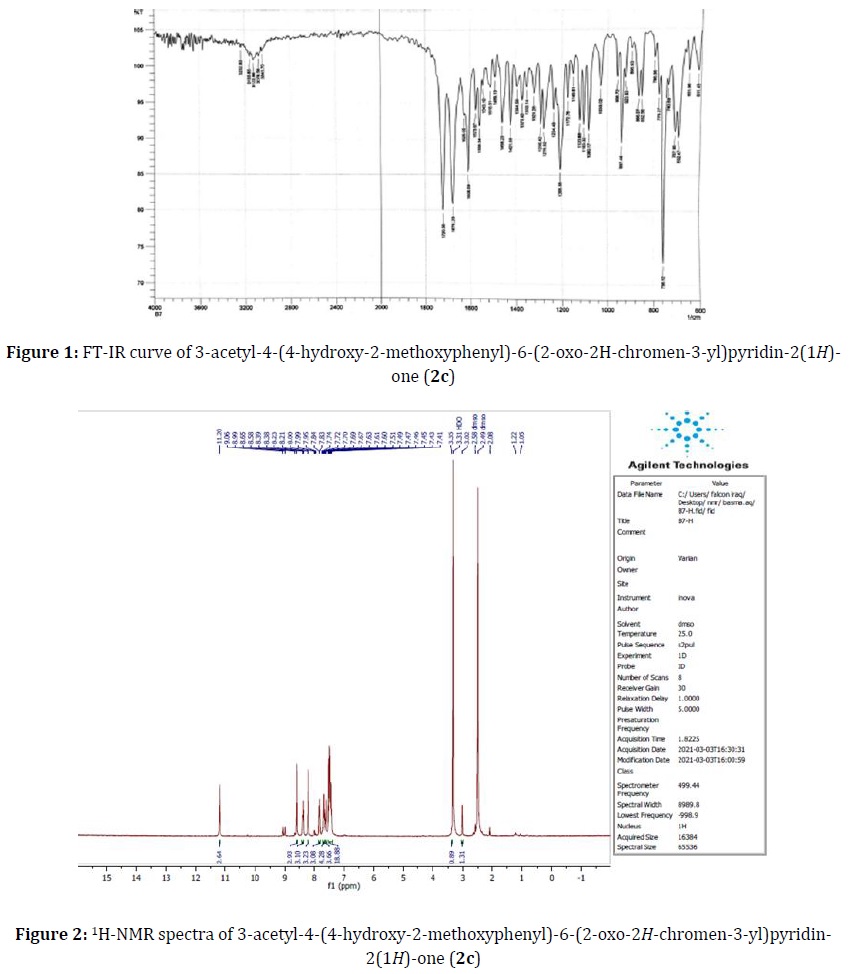
Pale yellow solid, yield 74%, mp 291-292 °C, Rf= 0.4, IR (KBr) (νmax/ cm-1): 3232 (OH), 3155 (NH), 1720, 1676, and 1626 (3C=O) cm-1 (Figure 1); (DMSO-d6, 400 MHz): δ 3.02 (s, 3H, CH3), 3.35 (s, 3H, OCH3), 7.41–8.95 (m, 9H, Ar-H in addition C=CH), 9.06 (s, 1H, OH), and 11.20 (s, 1H, NH) (Figure 2).
Samples’ preparation
Gamry instruments inc. provided mild steel specimens with a 2.89 cm2 active surface area and the following elemental arrangement employed as a basis for the following substrates for the gravimetric corrosion tests. The results are Fe 90.37, C 0.21, Si 0.40, P 0.017, S 0.002, Mn 0.12, and Al 5.16 (wt%). The checked coupons were cleaned using the ASTM standard procedure G1-03 [10]. All of the experiments were conducted with diluted hydrochloric acid made by diluting 37 percent HCl in water. The concentrations of 0.5 M hydrochloric acid and various pyridine derivatives (2a-c): The measuring solutions were 0.001, 0.002, 0.003, 0.004, and 0.005 M. A blank solution was employed (2a-c) in the absence of the pyridine derivatives, for comparison.

Scheme 1: inhibitor synthesis process
Weight loss measurements
Mild steel was used to make two square test specimens with measurements of 1.7 cm, 1.7 cm, and 0.1 cm and the chemical composition mentioned above. Samples were washed with distilled water using a clean tissue to dry, soaked in acetone in addition to alcohol, dried again, and stored in desiccators on a silica gel bed until needed. Each sample's measurements were carefully taken using a vernier that was weighted to the fourth decimal of a gram and set to the second decimal of a millimeter. In a conical flask, the metal specimens were totally submerged in 250 mL 0.5 M HCl solutions, uninhibited and inhibited. They were exposed to 25 °C and inhibitor concentrations for 3, 6, 12, 24, 48, and 72 hours. After that, the metal samples were cleansed, washed with purified water, and used a clean tissue to dry before being soaked with acetone and alcohol, and then they were dried. In the presence and absence of inhibitor, weight losses in mg cm-2 hour-1 were measured. To begin, at a dose of 0.001 M, all inhibitors were tested and a temperature of 25 °C to see which one was the most effective. The most efficient inhibitor was then tested at various temperatures (25–65 °C) and inhibitor concentrations of 0.001, 0.002, 0.003, 0.004, and 0.005 M.
Theoretical calculations
Using the Gaussian 09, edition A.02 software and a combination of the Lee-Yang-Parr (LYP) [11, 12] exchange functional and three-parameter hybrid (B3) exchange functional developed by Becke, all theoretical calculations for the compounds under investigation and the correlational and practical (B3LYP) were performed. The basis set 6-311G and a variation of the density functional theory (DFT) system are used to calculate every geometric optimization as well as the highest and the lowest molecular orbital energies (EHOMO and LUMO), respectively [13].
Results and Discussion
Chemistry
The synthesis of pyridine derivatives (2a-c) were carried out by refluxing 3-acetyl-2H-chromen-2-one with different aromatic aldehyde in ethanol [14-17]. The ethanol molecule is removed at the end of the reaction and the production of pyridine derivatives (2a-c) in 59-74% yield (Scheme 2).
Scheme 2: The reaction synthesis of pyridine derivatives (2a-c)
The physical properties of compounds 2a-c are listed in experimental section. The FT-IR spectrum of compounds 2a-c shows bands at the frequency of 1656-1626 cm-1 due to the carbonyl group of amide appearing and the NH near 3115-3188 cm-1. The 1H-NMR spectrum of compounds 2a-c demonstrates the following data: 2.3-3.02 for CH aliphatic and 10.5-11.2 for NH of the pyridine ring.
Weight loss tests
The weight loss technique is a well-known and widely used tool to determine the corrosion rates. Many studies have used it as an effective method to estimate metal loss [17–19].
The following equations (1), (2), and (3) were used to evaluate the values of corrosion rate (CR), inhibition efficiency (IE%), and mild steel surface coverage (θ) [20, 21]:


Where, wo and wi are the weight losses in the presence and absence of inhibitors, respectively. Mild steel coupons are losing weight was evaluated after different periods of exposure (3, 6, 12, 24, 48, and 72 hours) at various pyridine derivatives (2a-c) at 25 °C as indicated in Table 1 and inhibitor efficiency ranged from 0.1 to 36 percent, as depicted in Figure 3.
Pyridine derivative (2c) shows the higher performance which leads to a better understanding of the action of the pyridine derivative (2c).
In a corrosive setting at 25 °C, the pyridine derivative (2c) was found to minimize mild steel corrosion at low and high concentrations. Figure 4's plot of the corrosion rate vs. inhibitor concentration demonstrates that as the concentration of the pyridine derivative (2c) was raised, the corrosion rate (mmy-1) rapidly dropped.


The inhibitory performance improved as the concentration of the inhibitor was increased. At a concentration of 0.005 M of pyridine derivative (2c), the highest inhibition efficiency of 99.62 percent was obtained, as demonstrated in Figure 5. At all concentrations tested in this study, it was discovered that pyridine derivative (2c) reduces and controls mild steel corrosion. This is a result of molecules of the pyridine derivative (2c) adhering to the surface of mild steel. This adsorption decreases the dissolving of mild steel by obstructing corrosion sites and boosting inhibitive effectiveness.
Weight loss-temperature effect
Figure 6 displays the data for inhibitory efficiency at various temperatures (25, 35, 45, 55, and 65 °C). The results showed that the presence of pyridine derivative (2c) molecules reduced the rate of mild steel coupons corrosion in a corrosive environment. As seen in Figure 7, the inhibitory effectiveness increased as the concentration of Pyridine derivative (2c) rose. The inhibition efficiency was found to decrease as the temperature was raised, even though the pyridine derivative (2c) concentration was increased. This could be the result of the molecules of the pyridine derivative (2c) desorbing from the mild steel surface.
Surface characterization after immersion
For 24 hours at 25 °C, the scanning electron microscope was utilized to analyze the surface morphological observations of steel samples in an acidic environment with and without 0.001 M pyridine derivative (2c) molecules. The MS coupons exposed to hydrochloric acid corroded severely, as displayed in Figure 7. In contrast, adding pyridine derivative (2c) to the MS surface resulted in a soft surface with minimal damage. Figure 8 shows that in the presence of pyridine derivative (2c), the MS surface was sufficiently protected against corrosion [22].
Quantum chemical studies
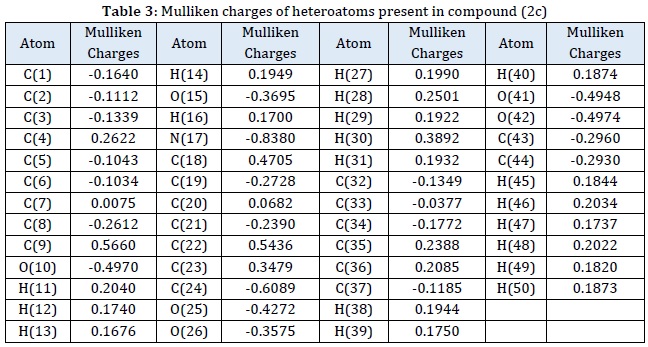
To learn more about the phenomenon of inhibition, theoretical calculations are quite beneficial. Theoretical factors such as HOMO, LUMO, ΔE, η, σ, χ, and ΔN are all linked to the inhibitor's inhibitory activation. These characteristics were discovered by the optimization of the inhibitor chemicals that have been studied [23, 24]. These parameters help to explain the dynamics of metal-inhibitor interactions. All quantum parameters for (2c) molecules were determined in this analysis. Table 2 provides an overview of the parameters' quantitative values.


Theoretical findings based on the study's proposed calculation method showed that compound (2c) is (-1432.01859a.u) more stable than other compounds (2A) and (2B) is (-1202.9959a.u) and (-1407.4783a.u) depending on the total energy. Figure 10 depicts the optimal geometry of (2c) inhibitor molecules. The inhibitor efficiency can be determined using the HOMO and LUMO energies.
![]()
he HOMO energy value shows how readily the inhibitor can give up electrons. Because of their great affinity for absorbing electrons from suitable acceptor molecules, inhibitor molecules are shown to have high HOMO values. The LUMO energy values suggest that the alloy can accept electrons from the inhibitor molecule. The inhibitor can acquire electrons from the alloy surface by back-donation at lower energy levels, according to LUMO. The energy gaps, ΔE (ΔE = ELUMO − EHOMO), reduced values indicate that the compounds have excellent inhibitory efficacy [25, 26]. A hard molecule has low basicity and low electron-donating potential, while a soft molecule has high basicity and high electron-donating potential, according to the hardness and softness notion [27, 28]. According to this finding, the inhibitor molecules' ability to restrict growth increases as they become softer while decreasing as they become harder. Two more significant characteristics that shed light on a material's stability and reactivity are its (hardness) and (softness) [29]. E has a low value for soft molecules but a high value for hard molecules. The low value of thus denotes a stronger corrosion inhibition efficacy [30, 31]. Table 2 shows that the E values for (2c) inhibitor molecules (2.6653eV), global softness and global hardness values as well as the proportion of electrons transported (N) for (2c) inhibitor molecules agree with the experimental findings. As reported in Table 2, the resulting value of χ = 4.5319 shows a large number of electron transfers, indicating that (2c) inhibitor molecules have the best inhibition efficiency. The number of electrons that a molecule may transfer to the acceptor molecule is determined by the number of electrons transferred (N) [32].
From the optimized molecular structure, the energies of the highest occupied molecular orbitals (EHOMO) and (ELUMO) were also computed. The dipole moment (μ), energy of the lowest unoccupied molecular orbital (ELUMO), employed in the computation, as well as the energy of the highest occupied molecular orbital (EHOMO) (ELUMO), the energy band gap (ΔEgap = ELUMO – EHOMO), the number of transferred electrons (ΔN), the ionization potential (I), and the electron affinity (A). According to density functional theory, the energy of the HOMO (EHOMO) is divided by the capacity of molecules to give metal electrons to determine the ionization potential. The greater the magnitude, the more capable molecules are in giving metal electrons. However, the electron affinity is inversely correlated with the LUMO energy (ELUMO), and a lower value means that electrons can reach the inhibitor from the metal surface more quickly. Concerning the transition complex's stability, the energy difference between HOMO and LUMO (ΔE), in turn describes how the adsorbed inhibitor interacts with the metallic substrate [33].
Mulliken Charges
Mulliken charges are crucial for determining the adsorption centers of the molecules being examined. The molecules under investigation have a strong negative charge, which accounts for their propensity to adsorb on MS surfaces [34]. In compound (2c) inhibitor molecules, nitrogen, oxygen, and some carbon atoms have larger negative charges, indicating coordinating interactions using the metal [35-38], as seen in Table 3 and Figure 9. As a result, the action centers were nitrogen and oxygen atoms, both of which may coordinate with MS's surface. 2c inhibitor compounds may take electrons from metal because of the positive charges on carbon atoms.


They are widely used by nucleophiles to form bonds. Superior inhibitors, according to some experts, have recently been shown to be able to interchange (give and accept) electrons with metal [39].
Conclusion
The following conclusions can be drawn from the current research:
- Successful production and evaluation of the three inhibitors as steel corrosion inhibitors in an acidic solution.
- The order of inhibition efficiency was 2C > 2B > 2A, according to the findings.
- Mild steel corrosion is effectively prevented by adding pyridine derivative (2c) to a 0.5 M HCl solution at different concentrations and inhibitor concentrations.
- Corrosion of mild steel is reduced when pyridine derivative (2c) is added to a 0.5 M HCl solution at various temperatures and inhibitor concentrations, and also inhibition efficiency diminishes as temperature rises.
- It was found that the quantum chemical values and the experimental results coincided. Researchers were able to examine the inhibitory effects of chemical compounds with similar structural features thanks to the recently developed Pyridine derivative (2c), which dramatically increased mild steel corrosion resistance.
Acknowledgements
The authors appreciate the facilities and ongoing support they have received from the Chemistry Division of the Department of Applied Science at the University of Technology (UOT).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
ORCID:
Hiba H. Ibraheem
https://orcid.org/0000-0002-7361-6266
HOW TO CITE THIS ARTICLE
Basma A. Hadi, Hiba H. Ibraheem. Experimental and Theoretical Studies on Corrosion Inhibition Performance of Pyridine Derivatives for Mild Steel in Acid Solution. Chem. Methodol., 2023, 7(2) 168-182