Document Type : Original Article
Authors
Department of Pharmaceutical Analysis, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences affiliated to The Tamilnadu Dr. M.G.R. Medical University,395, Sarojini Naidu Road, Coimbatore-641 044, India
Abstract
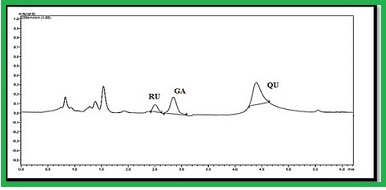
Sensitive and precise RP-HPLC method with photo diode array detector has been developed and validated for the simultaneous estimation of three commonly available anti-dengue, anti-cancer and anti-inflammatory phytochemical markers. The chromatographic separation was achieved using C18 column (250 mm × 4.0 mm, 5μm) with 0.2% v/v formic acid: acetonitrile (50:50; v/v) as mobile phase at the flow rate of 0.7 mL/min. The dual wavelength (280 and 360 nm) was selected for the identification and quantification of rutin, gallic acid and quercetin (Rt 2.56, 2.95 and 4.60 min). The method was validated as per ICH guidelines in terms of specificity, linearity, precision, and accuracy, LOD and LOQ, respectively. Linearity range for the selected markers, gallic acid, rutin and quercetin was found to be 1–10 µg/mL with the correlation coefficient value close to 1. The sensitivity of the method was demonstrated from the limit of detection which was found to be 0.4 µg/mL for gallic acid; 0.3 µg/mL for rutin and quercetin. The limit of quantification for gallic acid, rutin and quercetin was found to be 1 µg/mL, respectively. The % RSD and recovery values prove that the developed method was more precise and accurate. Hence, the proposed validated method has been successfully applied for the quality control analysis of gallic acid, rutin and quercetin in methanolic extract of Euphorbia hirta (L.) and Tawa-Tawa capsule formulation.
Graphical Abstract
Keywords
Main Subjects