Document Type : Original Article
Authors
- Lakshmi Jayanthi Juturi 1
- Rohinikumar Palavalasa 1
- Sneha H. Dhoria 2
- Subbarao Jampani 3
- Vijay Miditana 4
- Gouthami Jalem 5
1 Department of Chemical Engineering, R.V.R&J.C College of Engineering, Chowdavaram, Guntur, India
2 Department of Mechanical Engineering, R.V.R&J.C College of Engineering, Chowdavaram, Guntur, India
3 Department of Pharmaceutical Chemistry, Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Chowdavaram, Guntur, Andhra Pradesh, India
4 Department of Chemical Engineering, Centurion University of Technology and Management, Odisha, India
5 Department of Chemical Engineering, Government Polytechnic for Minorities, Guntur, India
Abstract
Urbanization and increased industrialization are both contributing to a very high level of stress on our water environment, which is reducing the supply of clean water. Water pollution affects the ecosystem and is a major concern for humans, flora, fauna, and the environment. Fluoride is a pollutant that is persistent and not biodegradable; it builds up in the soil, plants, animals, and people. Therefore, understanding its removal and using the best method with the greatest efficiency is required. Likewise, adsorption is an affordable and easy technique that could be adopted at the household level. The Sol-Gel process was used for the nano-MgO synthesis, which has a cost-effective recovery of the material. SEM, Fourier transform infrared spectroscopy, and X-ray diffraction analysis were done to characterize the synthesized nanomaterial. The produced nano magnesium oxide (nano-MgO) was evaluated for the defluoridation process. The results indicated that the occurrence of ions like bicarbonate and phosphate highly favors the adsorption of nano-MgO. Fluoride elimination using nano-MgO was achieved in this study by doing experiments in batches and considering three-time intervals. The optimal time for the utmost removal of fluoride was found to be 15 minutes, during which the concentration decreased from 8 ppm to 3.1 ppm.
Graphical Abstract
Keywords
Main Subjects
Introduction
There has been a growing interest in using advanced technologies like nanotechnology and nanostructures to address environmental issues. This has led to many studies investigating the potential of these technologies for a range of applications like wastewater treatment, clean energy production, environmental remediation, renewable, and energy storage.
Nowadays, proper wastewater management is crucial to protecting the environment and public health. Fluoride is one of the most common elements in the earth's crust. Fluoride is a water pollutant that can affect a variety of organisms, like frogs, fish, insects, and some phytoplankton. Water is crucial for both human survival and a country's economic growth [1]. Moreover, drinking the contaminated water poses a significant number of health issues, including cancer, skeletal and dental fluorosis, dwarfism, brittle bones, disorders of the kidneys, etc. [2]. The World Health Organisation (WHO) states the maximum contaminant level (MCL) of fluoride in the water that can be consumed should fall between 0.5 and 1.0 mg/L, with minor adjustments made for various geographical locations [3]. In many parts of the world, groundwater has greater concentrations of fluoride which is greater than 1.5 mg/L, necessitating treatment [4-7]. Fluoride is considered as one of the chief inorganic pollutants found in groundwater in India and is primarily of hydrogeochemical origin. It is found that high bicarbonate alkalinity and low calcium favour groundwater with high fluoride content [8,9].
In the past, various adsorbents have been employed to find a defluoridating agent that is both effective and affordable. Numerous adsorbents such as activated alumina, bauxite, rice husk, bone char, and magnesium-ended activated alumina have been tried. Among the different adsorbents used, the activated alumina technique for defluoridation from water is reckoned to be the most suitable sorbent. As per the World Health Organisation (WHO), fluoride is considered as suitable in drinking water at 0.7 mg/L but injurious once it exceeds 1.5 mg/L [10,11]. However, due to some limitations, such as optimal removal at a low pH value of 5.5, its real-world possibility of applicability is lessened. Many researchers have studied diverse economical adsorbents used for the removal of fluoride [12].
Fluoride is solitary common element in the present earth's crust. The physicochemical properties of fluoride are listed in Table 1. The range of fluoride in seawater is 0.86 to 1.4 mg/L, with a standard of 1.1 mg/L. The chloride concentration is about 19 g/L. In drinking water and foods, the fluoride presence is low, which reflects the insolubility of alkaline earth fluorides like calcium fluoride. In general, freshwater supplies have fluoride between 0.01 and 0.3 ppm.
Table 1: Phyisco-chemical properties of fluoride
|
Property |
Flouride |
|
Atomic number |
9 |
|
Atomic weight |
18.998 |
|
Chemical formula |
F− |
|
Melting point |
-219.6 0C |
|
Boiling point |
-188 0C |
In recent times, nanomaterials have become appealing for a diversity of purposes, including defluoridation, because of their unique physical and chemical properties. In comparison to the conventional methods, using nanomaterials for defluoridation has several benefits, including increased adsorption capacity, accelerated adsorption kinetics, and lower cost [13]. Nano-sized materials have a larger surface-to-volume ratio, increasing their adsorption capacity for fluoride ions. The small size of the particles also allows for faster diffusion of fluoride ions to the surface of the material, resulting in faster adsorption kinetics. Nanomaterials can be synthesized using various processes, like sol-gel, precipitation, and hydrothermal techniques. This allows for the optimization of the material properties, such as surface area, porosity, surface chemistry, and porosity to enhance their defluoridation efficiency [14-17].
Nanomaterials like carbon nanotubes, graphene oxide, and metal oxide nanoparticles have been noticed for the removal of pollutants from soil and water [18,19]. Furthermore, researchers have developed various nanomaterials and nanostructures that can remove contaminants from wastewater, including heavy metals, organic compounds, and pathogens [20,21]. Nanotechnology is also being investigated as a means of producing renewable and clean energy. For instance, the use of nanowires and nanotubes is being explored to improve the efficiency of solar cells [22]. Lastly, researchers are studying the development of nanomaterials that can store hydrogen at high densities for energy storage purposes [23].
While there are still challenges to overcome, the potential benefits of these technologies are significant and warrant further research and development. These advanced technologies have the potential to revolutionize environmental remediation, wastewater treatment, energy production, and storage in the coming years.
Mashad et al. [24] processed magnesium oxide particles by co-precipitation and studied the effects of pH, temperature, and the molar ratio of precursors on the reaction (magnesium nitrate). The researchers used their method to produce nanoparticles and nanorods with relatively high specific surface areas (231 m2/g for nanoparticles and 176 m2/g for nanorods) and particle sizes of 50 nm. The findings demonstrate that pH and a polyethylene glycol (PEG) template have an impact on particle morphology.
Devi et al. [25] investigation on the adsorption of fluoride from water, and regeneration studies revealed that 1M HCl was the finest eluent, capable of removing 95% of the fluoride, followed by NaOH (2 M), with 25% regeneration of the adsorbent to be regenerated.
Bharali and Bhattacharya [26] experimented by drying the leaves of neem, which was utilized as a bio-adsorbent in powder form to fluoride removal from groundwater. The results indicated that fluoride removal efficiency at 2 was 74.25% with duration of 300 minutes.
Ranjit et al. [27] evaluated the fluoride removal from groundwater by the MTC technique. It concludes that the MTC has a good capacity to remove fluoride with the optimal dose. By considering fluoride at 2 g/L and 5 mg/L with 360 minutes as the optimal contact time in pH 6-8, the adsorption capacity was at its peak.
Minju et al. [28] noticed that using a modification of the sol-gel process, magnesium oxide-coated magnetite nanoparticles were prepared. The defluoridation of water used for drinking is made viable by the adsorbent's exceptional fluoride scavenging capacity for concentrations less than 10 mg/L.
Recently, there has emerged an increase in interest in employing nanoparticles, most significantly magnesium oxide (MgO) or magnesium hydroxide (Mg(OH)2), for defluoridation. Nano Magnesium oxide has been recognised as a viable treatment for fluoride elimination. This article explores the adsorption feasibility and potential of nano-MgO for fluoride removal. By evaluating its performance as an adsorbent, the researchers aim to determine its suitability for practical defluoridation applications. Nano-MgO offers advantages such as a high surface area and reactivity, which enhance its adsorption capabilities.
By effectively removing fluoride from water sources, the potential negative impacts on the environment and human populations can be significantly reduced. Nano-MgO shows promise as an efficient fluoride removal option. Overall, this research underscores the importance of addressing water fluoridation challenges and emphasizes the significance of proper wastewater treatment. Using nano-MgO as an adsorbent for removal of fluoride, the study contributes to the development of sustainable and effective defluoridation techniques. The outcomes of this study have the potential to positively impact both the environment and the community's well-being.
Materials and methods
Preparation of Nano MgO by Sol-gel process
Adsorption is one of various techniques that are typically thought to be interesting due to its efficiency, practicality, simplicity of use, environment, ease of design, and economics. Affordable adsorbents that can effectively remove F around the neutral pH of drinking water are used. In India, a technique known as the Nalgonda process has been further developed from the precipitation/coagulation method in which F is removed as a CaF2 precipitate using lime and Al salts, followed by the elimination of remaining F in solution by co-precipitation with adsorption on to the precipitated Al(OH)3. Other various techniques include the Nalgonda technique, activated alumina, and ion exchange.
The different methods for the nano-MgO preparation are chemical vapour deposition, spray pyrolysis, sol-gel process, and colloidal process. In this work, the sol-gel technique is used as there are many advantages, like less cost, simplicity, synthesis at low temperatures, high quality, and purity of materials. The sol-gel process, a commonly used process for the synthesis of nano-MgO particles, emerges the growth of networks by the arrangement of colloidal suspension (sol) and gelation to produce a system in a continuous liquid phase [29].

Figure 1: Steps in the synthesis of nano-MgO and its characterization methods
Based on the type of precursors used, the sol-gel method can be divided into two categories: Alkoxide precursors and inorganic precursors such as nitrates, chlorides, and sulphides [29]. Here, metal alkoxides react with water in the presence of a base or acid to form a single-phase solution that develops into a rigid double-phase system made up of solid metal oxides and pores filled with solvent. MgO is frequently synthesized using the sol-gel process because of its capacity to yield monodispersed particles with a narrow size allocation under mild circumstances [30].
In this work, potassium permanganate, magnesium nitrates, and sodium fluoride (NaF) were procured from Ranbaxy Fine Chemicals Limited, India. A total of 1000 mg/L fluoride stock solution was prepared from NaF using distilled water, and the required concentrations of the samples were processed by successive dilutions of the stock solution. The methodology adopted and the characterization techniques are displayed in Figure 1.
Synthesis of nano-MgO
Nano Magnesium oxide was synthesized using magnesium nitrate (MgNO3.6H2O) as source material with NaOH (sodium hydroxide). All the chemicals used for this synthesis were purchased from Ranbaxy Fine Chemicals Limited, India, and used without further purification.
The typical experimental process and synthesis of nano MgO are depicted in Figure 2. 0.2 M of magnesium nitrate (MgNO3.6H2O) was dissolved in 100 ml of distilled water. 0.4M NaOH was added dropwise to the prepared magnesium nitrate (MgNO3.6H2O) solution while stirring with a magnetic stirrer continuously. The ratio of metal to hydroxide ions was maintained at 1:2. The mixture was stirred for 60 minutes at room temperature to form magnesium hydroxide without any agglomeration. Later, the mixture was endorsed to settle for 30 minutes, and a white precipitate of magnesium hydroxide formed in the bottom of the conical flask.

Figure 2: Synthesis of nano MgO by sol-gel process
The precipitate was filtered with the Whatman filter paper to collect the precipitate, and that precipitate was placed in a 250 ml glass jar, allowing it to dry for some time. 50 ml of ethanol was added to the precipitate and placed in the jar in a dryer for 24 hours. A temperature of 120 °C was maintained so that any water molecules present in the precipitate would evaporate and form small crystals. Now placed the precipitate in a muffle furnace and calcined at 400 °C for 4 hours, and then it was taken out of the furnace, cooled, and grinded into a white powder that is nothing but nanomagnesium oxide. The chemical reaction and chemical structure are given as follow:

Characterization of nano-MgO by SEM, FTIR and XRD
Nano- MgO size was determined by using SEM(Carl Zeiass SEM with Oxford EDAX) at Osmania University, Hyderabad and is as shown in the Figure 3 The results indicate the size of the particle found was Minimum: 72nm, Maximum: 1420nm and Average: 700 nm. The image indicate perhaps MgO nano particles quite polydisperse.
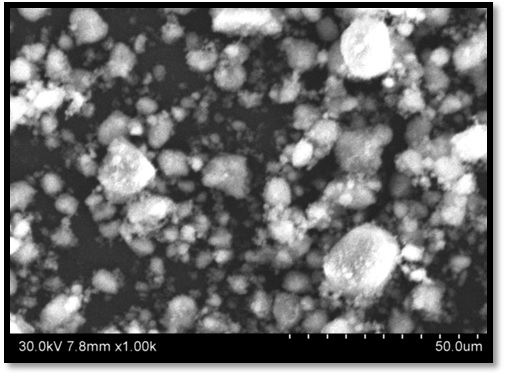
Figure 3: SEM micrographs of synthesized nano-MgO
Characterization of FTIR
Fourier transform infrared spectroscopy is a technique which is used to attain an infrared spectrum of absorption or emission of a solid, liquid or gas. FTIR test is conducted on Fourier transform Infrared Spectrometer (Make: Bruker Optics, Germany, Model: Tensor 27), in Osmania University, Hyderabad, the results are as shown in the Figure 4 FTIR Spectra of MgO particles are shown Due to moisture, there were peaks at 3664 cm-1 and 3448 cm-1 that correspond to the hydroxyl groups' O-H stretching mode.
Characterization by XRD
X-Ray diffraction analysis is a non-destructive process which gives detailed information about the chemical composition, crystallographic structure, and physical properties of a material. It only requires preparation of a minimal sample for analysis. Interpreting the resulting data is relatively straight forward. From the above graph, we can observe sharp peaks in both results. The peaks in the XRD graph show the presence of crystal structure so the structure of the particles is of crystalline nature from the above Figure 5 From the above characterization results it is concluded that the prepared particles are nano-MgO. MgO nanoparticles have XRD peaks at 42 (200 plane) and 62 (220 plane) so Figure 5 is showing evidence of that.
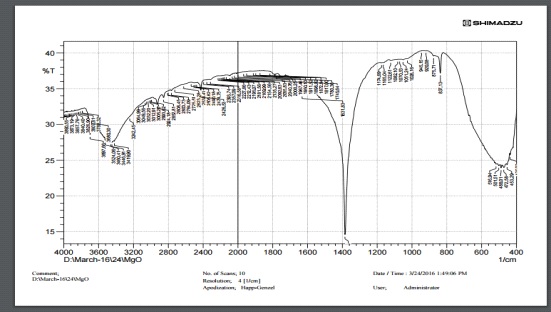
Figure 4: Result of Fourier transform infrared spectroscopy

Figure 5: XRD
Batch Adsorption Design
The experiments were conducted in batches using nano-MgO in various dosages ranging from 10 to 70 mg. A 100 mL fluoride solution with an initial absorption of 8 mg/L (ppm) was prepared in a 250 mL Erlenmeyer flask. The flask was placed on a magnetic stirrer at ambient temperature to ensure proper mixing. The adsorption process was allowed to proceed, and samples were collected at regular intervals of 15, 25, and 35 minutes.
After the adsorption process was completed, the samples were filtered using Whatman filter paper to eliminate any solid particles. The residual concentration of fluoride in the filtered samples was then analysed using a fluorimeter. Specifically, a Hach DR-900 fluorimeter was used for this purpose in this work.
To maintain the pH of the fluoride solution, 0.1 M HCl and 0.1 M NaOH solutions were engaged. This ensured that the pH remained within the desired range throughout the experiment, minimizing any potential impact on the adsorption process.
The batch adsorption studies were carried out meticulously to reduce the possibility of errors in the collected data. This approach allowed for better control and accurate evaluation of the fluoride removal efficiency using nano-MgO as the adsorbent.
Results and Discussion
The results obtained from the experiments performed and depicted in the graphs indicate that the optimum time required for achieving the maximum removal of fluoride is 15 minutes. During this period, the adsorption process reaches its peak efficiency, resulting in a significant reduction in fluoride concentration. This finding is crucial as it provides valuable information on the optimal duration needed for the effective fluoride removal.
Furthermore, the results (Figure 6) reveal that the optimum dosage of the adsorbent is specifically 50 mg, and corresponds to the point where the maximum removal of fluoride occurs. At this dosage, the adsorbent exhibits its highest adsorption capacity, resulting in a substantial decrease in fluoride concentration. This finding highlights the importance of dosage optimization to maximize the efficiency of fluoride removal process.
The reduction in fluoride concentration from 8 ppm to 3.1 ppm demonstrates the effectiveness of the chosen time and dosage parameters. It indicates a substantial removal of fluoride ions from the solution, which is crucial for ensuring the safety and quality of the water. The significant decrease in concentration underscores the potential of adsorption process and its ability to successfully mitigate fluoride contamination.
Overall, these findings emphasize the importance of considering both the optimum time and dosage in achieving efficient fluoride removal. By determining the optimal conditions, such as a 15-minute contact time and a dosage of 50 mg, the adsorption process can be optimized to achieve the maximum fluoride removal, resulting in a significant reduction in fluoride concentration from 8 ppm to 3.1 ppm. Table 2 indicates the comparison of earlier works by different researchers.
Gebremariam et al. [7] used activated carbon corncob and sorghum husk, modified with aluminium hydroxide, and the material showed promise for removing fluoride from water. Optimal conditions include a 0.063 mm adsorbent size, pH 7, 60 minutes contact time, and a dosage of 6 g. FTIR characterization confirms the interaction between activated carbon and fluoride, and electrostatic interactions play a role.
Manjunatha et al. [32] investigated the rapid and effective removal of fluoride using barium-doped calcium aluminate (BCA) nanoparticles. The BCA nanoparticles were characterized, and optimal conditions for pH, dosage, contact time, and adsorbate strength were determined. The adsorbent demonstrated efficient fluoride removal even in the presence of competing anions. Overall, this study establishes BCA nanoparticles as an effective adsorbent for groundwater fluoride removal.

Figure 6: Fluoride removal at different times
Table 2: Comparison of various nanoparticulate adsorbents
|
Ref. No |
Author |
Adsorbent |
Technique |
Characteristics |
Conclusions |
|
Gaurang Patel et al. |
Cao nanoparticles |
Sol-gel |
FT-IR, TGA, DTA, and TEM analysis |
98% of fluoride was obtained within 30 minutes. |
|
|
Xiaoli Zhao et al. |
Fe3O4@Al(OH)3 |
Chemical coprecipitation methods |
SEM, XRD, and thermal |
Fluoride-sensitive adsorbent with high adsorption capacity. |
|
|
Proma Bhattacharya et al. |
Nano magnesium oxide |
Aqueous extract |
TEM, DLS, and XRD |
Concentration 5 mg.ml-1 |
|
|
Hanif Amrulloh et al. |
Magnesium oxide nanoparticles |
Aqueous extract |
SEM, TEM, and XRD |
Moleifera bark and MgO nanoparticles have good antibacterial activity against S. aureus, E. faecalis, E. coli, and S. dysenteriae with MIC values (300 - 550 g/mL) |
|
|
S. Rajeshkumar et al. |
Copper nanoparticles |
Eco-friendly green |
XRD, UV–Vis spectroscopy, FT-IR, TEM, and SEM |
Excellent antimicrobial activity |
Conclusion
In conclusion, this study demonstrated that nano magnesium oxide (nano-MgO) is an effective adsorbent for the removal of fluoride from water. The synthesized nano-MgO was characterized using SEM, XRD, and FT-IR, which confirmed its purity and provided information about its crystal structure, surface morphology, and chemical composition.
The fluoride adsorption experiments were done by varying the adsorbent dosage and the initial fluoride concentration with time. The results show that the optimum time for fluoride adsorption was 15 minutes and the optimum dosage of nano-MgO was 50 mg. The adsorption efficiency was found to be high, indicating that nano-MgO has a significant potential for removing fluoride from contaminated water.
Overall, this study suggests that nano-MgO could be a promising material for the defluoridation of water, particularly in areas where fluoride contamination is a significant concern. Further research could focus on exploring the feasibility of using nano-MgO on a larger scale and optimizing the adsorption process to enhance its efficiency and effectiveness in real-world applications.
Acknowledgements
The authors acknowledged and appreciated everyone facilitating this investigation to be achieved.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the article and agreed to be responsible for all the aspects of this work.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work
ORCID
Lakshmi Jayanthi Juturi
https://orcid.org/0000-0002-2771-2783
Rohinikumar Palavalasa
https://orcid.org/0000-0001-6741-8143
Sneha.H.Dhoria
https://orcid.org/0000-0002-1131-1248
Subbarao Jampani
https://orcid.org/0000-0001-6984-2423
Vijay Miditana
https://orcid.org/0000-0002-9226-8711
Jalem Gouthami
https://orcid.org/0009-0007-1342-5760
HOW TO CITE THIS ARTICLE
Lakshmi Jayanthi Juturi, Rohinikumar Palavalasa, Sneha H. Dhoria, Subbarao Jampani, Vijay Miditana, Gouthami Jalem. Efficient Removal of Fluoride Using Sol-Gel Processed Nano Magnesium Oxide. Chem. Methodol., 2023, 7(7) 569-580


