Document Type : Review Article
Authors
1 Department of Medicinal Chemistry, School of Pharmacy, Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2 Department of Pharmaceutics, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
The use of polymers modified with folic acid in drug delivery to treat many diseases has long captivated the attention of scientists. Given the distinctive characteristics of folic acid in the realms of food, pharmaceuticals, and chemicals, this review explores the methods employed in bonding folic acid to diverse polymers through the lens of organic synthesis. Furthermore, it examines the behavior of folic acid that has been conjugated to different polymers in drug delivery, along with the interaction between folic acid and folate acceptors. It is important to acknowledge that the articles employed in this review span the years 2002 to 2023.
Graphical Abstract
Keywords
Main Subjects
Introduction
The significance of targeted drug delivery in the procedure of managing various diseases is widely recognized [1-4]. Presently, scholars are contemplating the development of optimal drug delivery systems from multiple perspectives, rapid cellular uptake, enough toxicity consequences, appropriate drug release, and numerous other considerations [5-9].
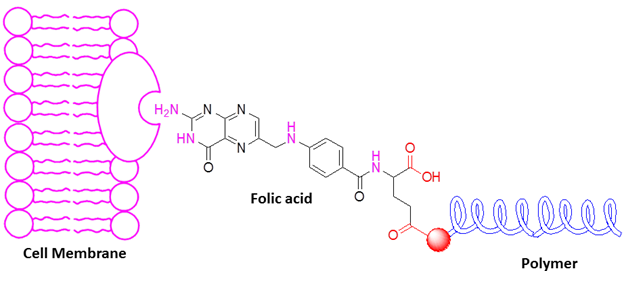
The utilization of polymers as carriers for targeted drug delivery has instigated a great upheaval within the domain of drug delivery [10,11]. The conferment of attributes such as enhancing drug solubility, augmenting drug loading capacity, regulating the duration of drug circulation within the bloodstream, diminishing drug toxicity towards healthy cells, and amplifying drug toxicity towards tumor tissues through the utilization of polymer-carriers is deemed a momentous advancement within the realm of drug delivery [12-17]. Given that, the primary objective of targeted drug delivery is the precise and suitable delivery of pharmaceuticals to living cells, the utilization of systems that function as pioneers facilitates the realization of this objective [18-21]. In recent times, biologically active molecules like vitamins, growth factors, and hormones have presented a novel avenue for researchers to exploit natural endocytosis pathways through their interaction with drug-encapsulating polymers [22,23]. The overexpression of folate receptors on the surface of cancer cells introduces folic acid as a suitable option for the leadership of drug-delivery polymer systems. It is essential to highlight that the distribution of the folate receptor is restricted in normal tissues [24,25].
Folic acid, a member of the B vitamin family commonly referred to as vitamin B9, is an organic molecule. Within its molecular structure, folic acid possesses functional groups that exhibit activity and can form covalent bonds with numerous polymers through interaction with functional groups [26-28]. The pharmaceutical and food industries have embraced the multifaceted applications of this compound, thereby endowing it with a distinct and significant standing within the realm of pharmaceuticals [29]. According to the significance of folic acid within modern drug delivery systems [30], the present review endeavors to acquire a precise comprehension of the methods employed for synthesizing polymers that have been modified with folic acid. Furthermore, the conduct of folic acid when bonded to various kinds of drug delivery polymers shall be scrutinized and a thorough examination of the interaction between folic acid and its receptor, accomplished through an extensive review of published literature.
Synthesis of Folic Acid Conjugated With Carrier Polymers
Folic acid possesses multiple functional groups that offer the necessary potential for interaction with diverse polymers. As depicted in Scheme 1, folic acid encompasses two carboxylic acid groups and several amine groups. The carboxylic acids exhibit heightened reactivity in comparison to the amino groups, rendering folic acid more active at the carboxylic acid terminal. Within the two carboxylic acid groups, the second group is more conveniently accessible due to reduced steric hindrance and possesses a suitable capability for bonding [31,32]. An enhanced understanding of the bonding of folic acid to polymers can be attained by examining the synthetic aspects discussed in subsequent sections. In addition, the bonding of folic acid to polymers can be broadly categorized into two facets, namely direct and indirect bonding to the polymers.
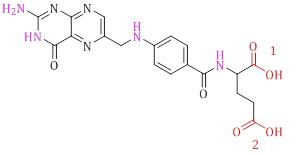
Scheme 1: Molecular structure of folic acid
Direct Boding
In the process of direct type folic acid undergoes covalent bonding with the respective polymer, thereby indicating that the polymer possesses appropriate functional moieties for interacting with folic acid. Subsequently, the section highlights the synthetic approaches employed by studies investigating the direct bonding method.
Pasut et al. achieved successful synthesis of Folate-PEG-COOH by employing carboxylic acid activating reagents, namely N-hydroxysuccinimide (NHS), to facilitate the bonding of folic acid to the polyethylene glycol (PEG). The central focus of their investigation lies in the functionalization of the polymer, enabling its interaction with folic acid (Scheme 2) [33].
The utilization of the esterification reaction to bond folic acid to the polymer is one of synthesis methods of functionalized polymers with folic acid. Lee et al. synthesized folic acid-conjugated pullulan/poly(DL-lactide-co-glycolide) using carboxylic acid activating reagents such as DCC and DMAP (Scheme 3).
The novelty in the synthesis aspect of their study lies in the positioning of folic acid on the side branches of the polymer, as opposed to the polymer terminus [34].
Beagan et al. developed Poly(2-hydroxyethylmethacrylate-co-2-folateethylmethacrylate) (HEMAF) using the solution casting method. The distinguishing feature of their approach is the reaction of folic acid with monomer units, which occurs during the subsequent polymerization phase. With this method, a greater quantity of folic acid is bound to the polymer (Scheme 4) [35].
In a general sense, it is crucial to devote considerable attention to synthetic aspects in the direct bonding of folic acid to polymers. Primarily, the investigated polymers contain functional groups NH or OH [33,36].
Secondly, the position of the mentioned functional groups instigates either terminal or branching ramifications within the final polymer. Lastly, if the NH or OH groups are absent, it becomes imperative to modify the polymer's structure.
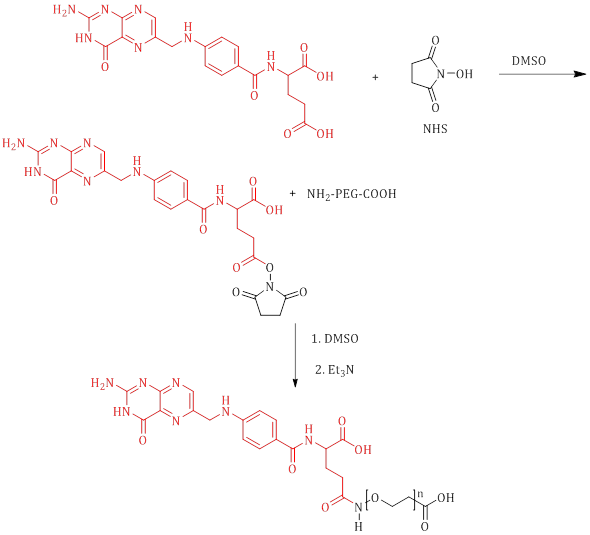
Scheme 2: Synthesis of Folate-CONH-PEG-COOH
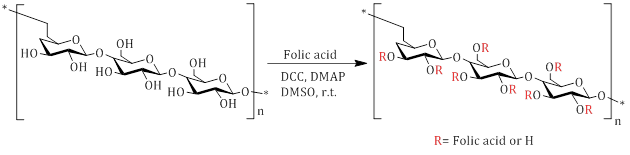
Scheme 3: Synthesis pathway for Folic-acid-conjugated pullulan/poly(DL-lactide-co-glycolide)
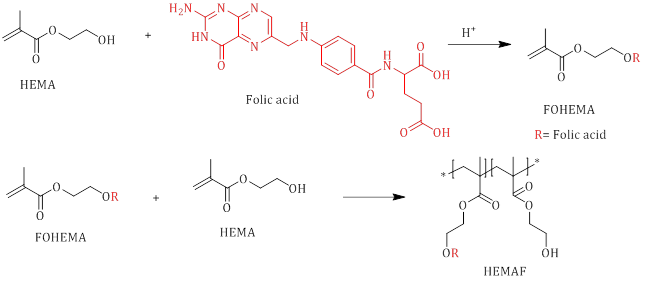
Scheme 4: esterification reaction between 2-hydroxyethylmethacrylate (HEMA) and folic acid and synthesis of HEMAF via copolymerization reaction between HEMA and FOLEMA monomers
Indirect Bonding
In the indirect category, the relevant polymer lacks functional groups that are appropriate for forming bonds with folic acid, necessitating the utilization of compounds as a means of linkage. It is essential to acknowledge the advantages of bonding folic acid to polymers due to the extensive variability in their structures. In light of this, numerous investigations have been carried out to employ linkage to establish a connection between folic acid and polymers, some of which are discussed below.
In 2011, Sulistio et al. conducted research on the development of intriguing star polymer systems utilizing poly(L-lysine)armpoly(L-cystine)core PEG and folic acid. Specifically, PEG serves as a linkage between folic acid and the star polymer. Scheme 5 illustrates the formation of a bond between modified PEG, possessing terminal SH, and folic acid through an esterification reaction [37].
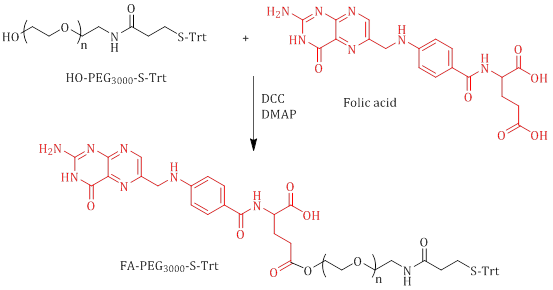
Scheme 5: Synthesis of FA-PEG3000-S-Trt
In addition, Figure 1 outlines the overall procedure for synthesizing star polymers in a schematic manner.
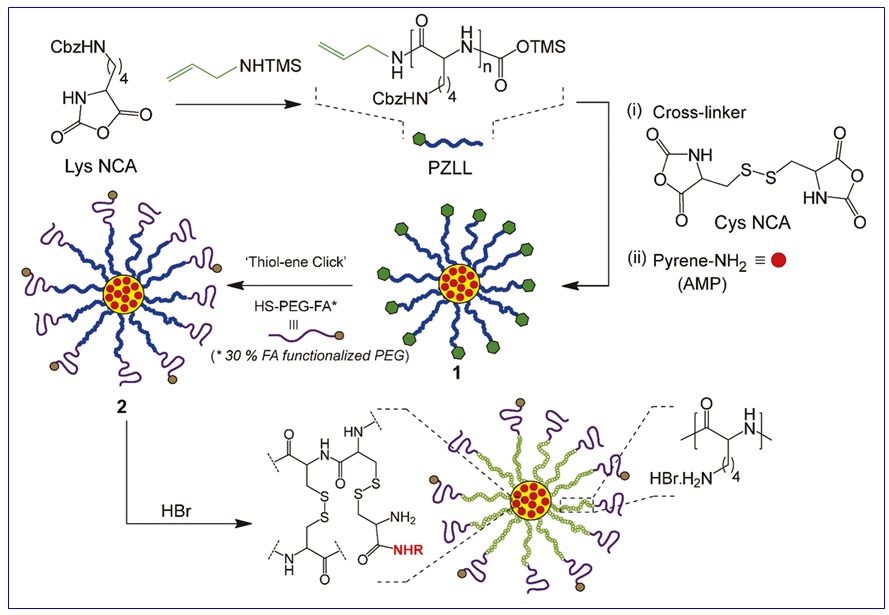
Figure 1: Synthesis of amino acid-based star polymers
Qiang et al. conducted the synthesis of fluorescent polymer nanoparticles by employing polyethyleneimine (PEI) as a linkage for the conjugation of folic acid. The reaction mechanism involving the polymer, PEI, and folic acid is visually represented in Figure 2. EDC and NHS were used as activators of carboxylic acid group [38].

Figure 2: Preparation of folic acid-functionalized fluorescent nanoparticles (FANPs)
Using diamine derivatives presents an intriguing concept to establish a connection between folic acid and various polymers. Pillai et al. employed ethylene diamine to bond folic acid to an acrylic polymer. Figure 3 illustrates the sequential stages involved in the synthesis of the folic acid-conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel [39].

Figure 3: Synthesis of folate conjugated cross-linked acrylic polymer hydrogel
In general, in indirect synthesis, the use of amine derivatives and modified intermediate polymers to bond folic acid to the corresponding polymers are suitable solutions for the synthesis of targeted drug delivery polymers [40-43].
The Role of Folic Acid on Effective Factors in Drug Delivery
The examination of the role of folic acid in drug-carrying polymers can be explored from multiple perspectives. Numerous scholars [44,45] have investigated the function of folic acid through the execution of diverse experiments in the realm of drug delivery, which will be discussed subsequently.
Quintana et al. conducted a captivating investigation on the structure of a therapeutic nano-device based on dendrimer-folic acid for methotrexate delivery to tumor cells. In the biological evaluation of the devised model, they employed the KB cell line, which prompts the overexpression of folate. The outcomes of the drug delivery exhibited that their nano-system exhibited a fourfold increase in efficacy compared to the free drug system. Flow cytometry data in Figure 4 shows the efficiency of conjugated polymer and folic acid in intracellular drug delivery [46].
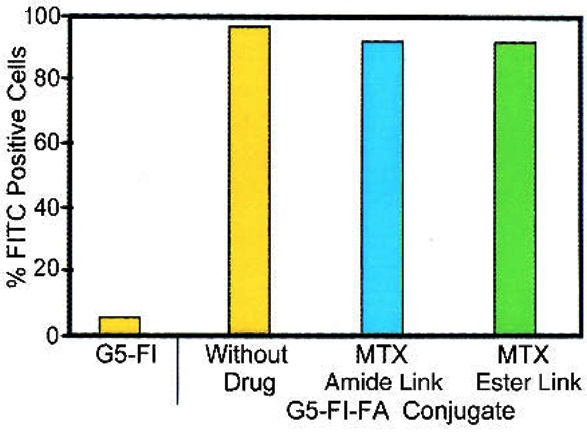
Figure 4: Flow cytometry data for uptake KB cells, methotrexate-dendrimer (G5-FI) [32]
Pasut et al. formulated PEG-gemcitabine prodrugs that are specifically targeted by folic acid. The objective of this was to address the issues of gemcitabine delivery, such as its short plasma half-life, rapid metabolism, and limited selectivity towards tumor tissue. The findings of their research indicated that the conjugated system was capable of releasing gemcitabine in a pH-dependent manner, with no involvement of enzymes. The pharmacokinetic profiles were found to be closely associated with the molecular weight of the polymer, and the inclusion of folic acid targeting enhanced the affinity towards cells that over-express folic acid receptors by 2-3 times.
Table 1 presents the antiproliferative activity against various cell lines, including HL-60, Hela, HT-29, MCF-7, and KB-3-1. By analyzing the data presented in Table 1, significant results can be obtained, such as the fact that the conjugates enter the cells through slower endocytosis pathways compared to the free diffusion of the drug.
Furthermore, it can be observed that the activity of the conjugates is only evident once the drug is released from the polymer. In the case of the targeted conjugates 4 and 5, which were tested on the KB-3-1 cell line that over-expresses folic acid receptors, they were found to be less cytotoxic than gemcitabine but more cytotoxic than the non-targeted product. Thus, it can be concluded that the targeted conjugates exhibit greater selectivity in hitting the KB-3-1 cells compared to the non-targeted 1 conjugate or free gemcitabine, thereby highlighting the significance of folic acid as a targeting moiety [33].
Table 1: IC50 values (μM) in different cell lines
|
No. |
Compound |
HL-60 |
Hela |
HT-29 |
MCF-7 |
KB-3-1 |
|
0 |
Gemcitabine |
0.028 |
0.024 |
0.084 |
0.46 |
0.41 |
|
1 |
PEG5000- Gemcitabine |
0.085 |
0.033 |
0.40 |
0.66 |
2.01 |
|
2 |
PEG20000- Gemcitabine |
0.067 |
0.026 |
0.20 |
0.58 |
- |
|
3 |
PEG220000- Gemcitabine |
0.092 |
0.066 |
0.62 |
0.73 |
- |
|
4 |
Folate-PEG4800- Gemcitabine |
- |
- |
1.45 |
- |
1.46 |
|
5 |
Folate-PEG4800- Aminoadipic Acid- Gemcitabine |
- |
- |
1.58 |
- |
1.13 |
In 2009, Chen et al. developed a platform comprising of liposomes targeted towards folate receptors to augment the effectiveness of the anti-cancer properties of arsenic trioxide (As2O3). The variables explored by the researchers encompassed the uptake of these arsenic liposomes by cellular structures and their antitumor efficacy in folate receptor (FR)–positive KB and HeLa cells, as well as FR-negative MCF-7 tumor cells. Their investigation was carried out using confocal microscopy, inductively coupled plasma mass spectroscopy, and cytotoxicity studies. The uptake of folate-targeted liposomal arsenic by KB cells was found to be three to six times higher compared to that of free As2O3 or non-targeted liposomal arsenic. This enhanced uptake was observed to occur through folate-mediated endocytosis, resulting in a 28-fold increase in cytotoxicity. In contrast, tumor cells with a lower density of FR on their surface, such as HeLa and MCF-7 cells, exhibited significantly lower uptake of the folate-targeted drug and consequently, lower efficacy.
In cocultures of KB and MCF-7 cells, the folate-targeted arsenic liposomes were exclusively internalized by KB cells, thus revealing a notable degree of targeting specificity. The confocal micrograghs confirm the cellular uptake data according to the intensity of the colors (Figure 5) [47].
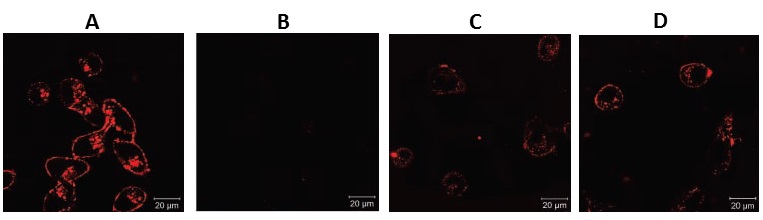
Figure 5: Confocal micrographs, A) folic acid-liposome-As2O3 for KB cells, B) Liposome-As2O3 for KB cell, C) folic acid-liposome-As2O3 for Hela cell, and D) folic acid-liposome-As2O3 for coculture [33]
Zhou et al. developed a notable formulation consisting of chitosan/alginate on poly(lactide-co-glycolide)-folic acid-targeted nanoparticles with the purpose of selectively targeting cells. They used flow cytometry and confocal laser scanning microscopy (CLSM) to investigate the impact of the nanoparticles' surface chemistry on their cellular uptake. Especially, HepG2 cell line exhibited significantly lower uptake of chitosan/alginate-coated nanoparticles compared to the bare nanoparticles, but the uptake increased upon the attachment of folic acid molecules.
Figure 6 indicates the percentages of uptake over time. It is significantly that the uptake ratio of free nanoparticles surpasses that of all other systems, and subsequently, the polymer system conjugated with PEG and folic acid exhibits greater cellular uptake. It should be mentioned that all polymer systems contain a specific drug concentration and exhibit slow release, resulting in a decelerated cellular uptake [48].
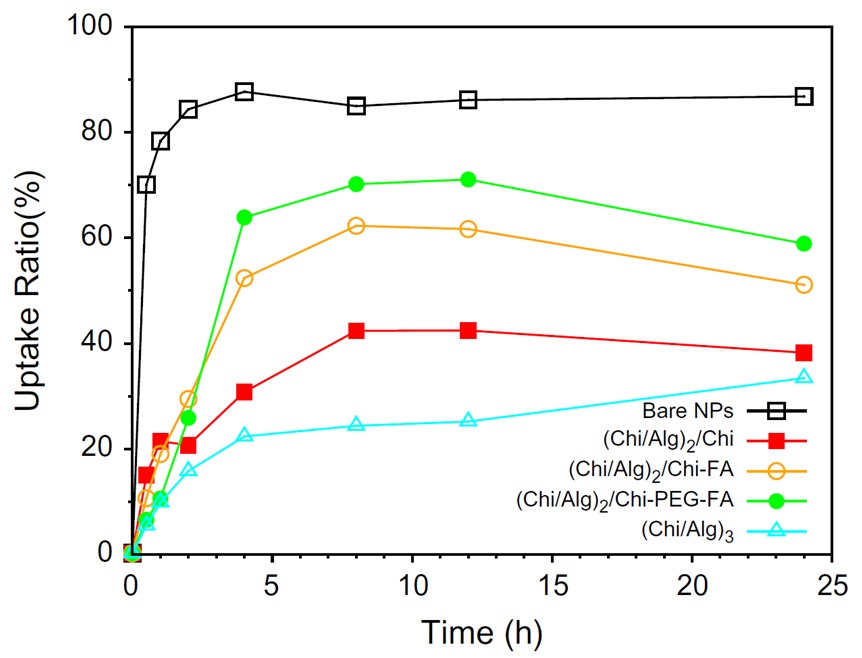
Figure 6: Cell uptake ratio for bare NPs, (Chi/Alg)2/Chi, (Chi/Alg)2/Chi-FA, (Chi/Alg)2/Chi-PEG-FA, and (Chi/Alg)3 [34]
The conjunction of folic acid with star polymers, which are based on amino acids, has generated a novel advancement in the field of drug delivery systems. Sulistio et al. conducted the synthesis of polymers known as core cross-linked star (CCS) polymers, where the core is composed of poly(L-lysine) and poly(L-cystine), functionalized with PEG and folic acid. To monitor the movement of star polymers, a fluorophore was utilized in the in vitro incubation of the polymers with breast cancer cells (MDA-MB-231).
The application of confocal microscopy and flow cytometry allowed for the determination that the cells were capable of internalizing the star polymers, with a higher degree of internalization observed in the presence of folic acid moieties. The presence of red dots in Figure 7 (F and D) indicates the uptake of the polymer-PEG-folic acid into the nucleus (blue) and cell membrane (green) [37].
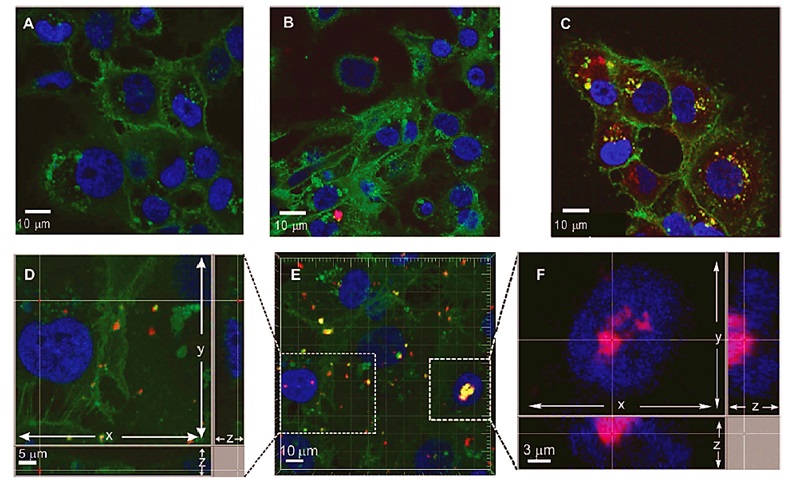
Figure 7: Confocal microscopy images A) Untreated MDA-MB-231 cells, B) Incubated cells with polymer-PEG, and C, D, E, and F) Incubated cells with polymer-PEG-folic acid [25]
The concept of synthesizing fluorescent polymer nanoparticles that are conjugated with folic acid to target cancer cells was conducted by Qiang et al. (2012). The quantitative results based on fluorescent polymer-methacrylamide-folic acid obtained from flow cytometry analysis on the cell lines HepG2 and HeLa are visually displayed in Figure 8. Despite the fact that HepG2 is classified as a human hepatocellular carcinoma cell line, it exhibits a negative response to FR (folate receptor). The expression of FR proteins in HepG2 cells is observed to be at a relatively low level.
The experimental results obtained from flow cytometry demonstrated that HeLa cells have the capability to uptake folic acid-labeled nanoparticles approximately four times more efficiently than HepG2 cells [38].
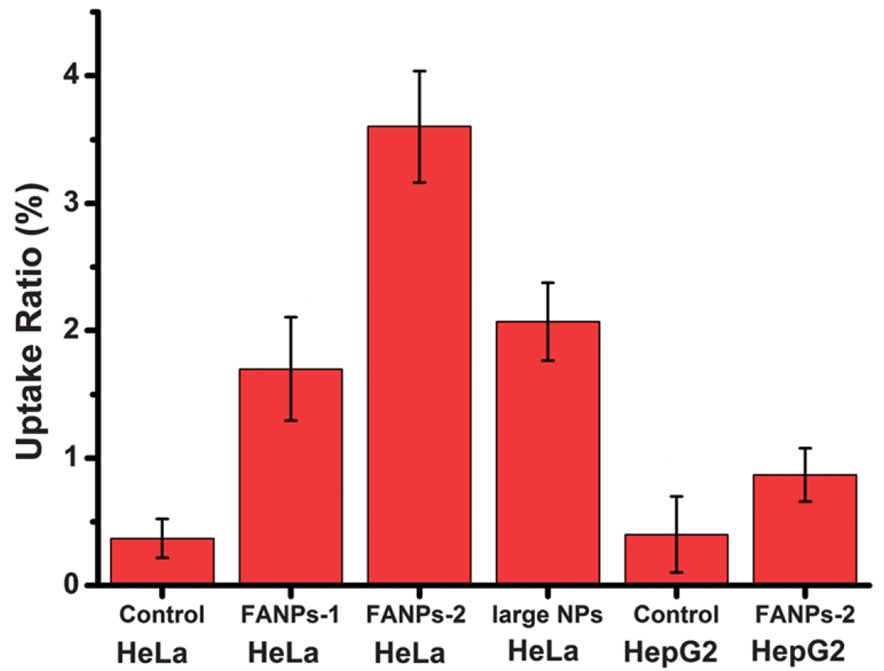
Figure 8: Uptake ratios of HeLa and HepG2 cells towards NPs measured by flow cytometry for control, folic acid-nanoparticles (folic acid/polyethyleneimine: 15/1) (FANPs-1) and folic acid-nanoparticles (folic acid/polyethyleneimine: 30/1) (FANPs-2) [26]
Nair et al. used a folic acid conjugated d-Valerolactone-Poly(ethyleneglycol) based triblock copolymer as a highly promising carrier for the targeted delivery of doxorubicin [49]. They conducted an assessment of the cellular uptake and cytotoxicity of polymer micelles in the presence and absence of folate on the folate receptor-positive breast cancer cell line, MDAMB231. The graphical model of cell uptake is presented in Figure 9.
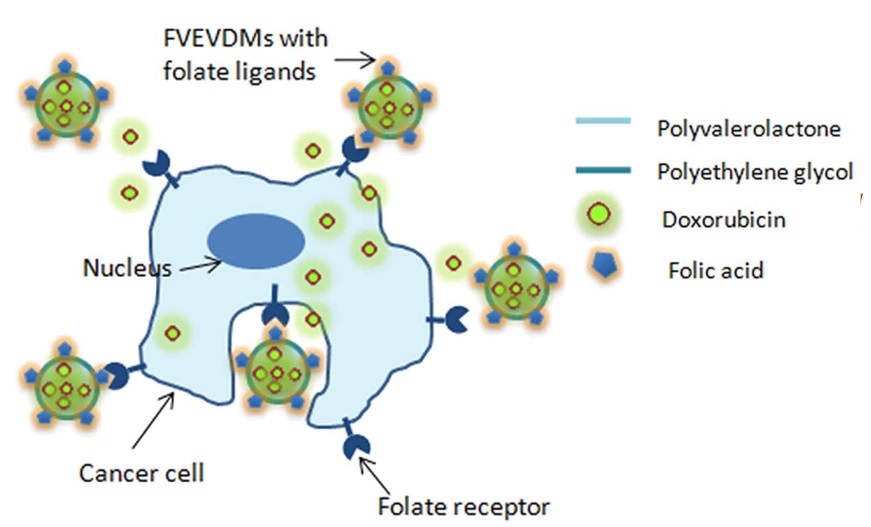
Figure 9: Graphical model of cell uptake d-Valerolactone-Poly(ethyleneglycol)-folic acid [35]
Confocal microscope images show that the intensity of red points in folic acid- Valerolactone-Poly(ethyleneglycol) is significant (Figure 10). The intracellular uptake experiments revealed a considerable enhancement in the uptake of folate micelles when compared to non-folate-mediated micelles. The outcomes of their study highlight the potential of the folic acid-labeled copolymer as a promising biomaterial with controlled and sustainable tumor targeting capabilities for anticancer drugs, thereby potentially opening up new avenues in the field of targeted chemotherapy.
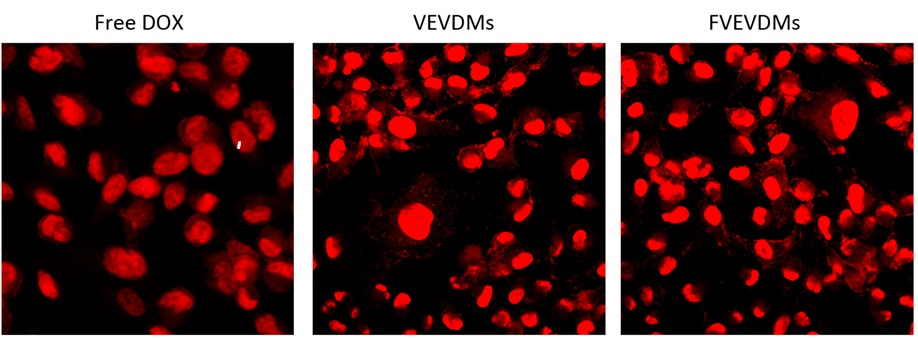
Figure 10: Confocal microscop images for free doxorubicin (Free DOX), Valerolactone-Poly(ethyleneglycol) (VEDMs), and folic acid- Valerolactone-Poly(ethyleneglycol) (FVEVDMs) [35]
The implementation of poly(ethylene glycol)–chitosan oligosaccharide lactate conjugated with folic acid for targeted ovarian cancer to deliver siRNA was conducted by Li et al. The process of folic acid grafting considerably enhanced the cellular uptake of nanoparticles through receptor-mediated endocytosis. Their research mainly focused on evaluating the accumulation efficiency of designed nanoparticles in BALB/c mice with OVK18 #2 tumor xenograft using in vivo imaging (Figure 11). The functionalized nanoparticles, which exhibited active targeting, demonstrated significantly higher accumulation than no targeted nanoparticles. After 12 and 24 hours, the concentration of functionalized polymer with folic acid is higher than without folic acid [43].
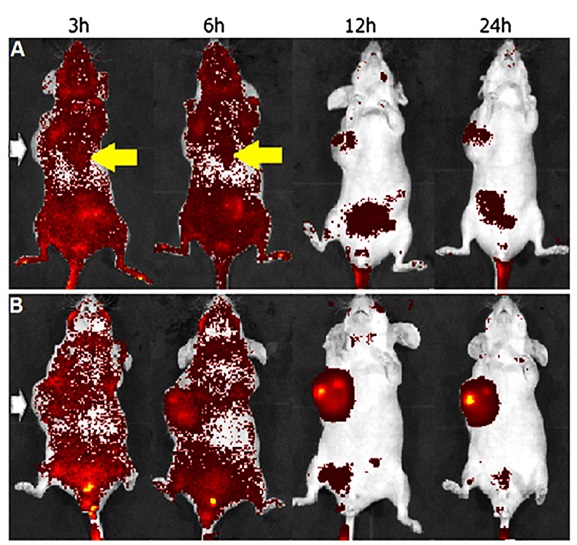
Figure 11: In vivo NIR fluorescence images of nude mice for A) Targeted polymer and B) No targeted polymer. White arrow indicates location of tumor xenograft and yellow arrow indicates liver accumulation [31]
The investigation conducted by Pillai et al. (2014) focused on the utilization of hydrogel systems for the delivery of hydrophobic drugs [39]. To facilitate the delivery of curcumin [50,51], they devised a hydrogel known as folic acid conjugated cross-linked acrylic polymer (FA-CLAP). The researchers examined the drug's release kinetics from the entrapped state, which displayed an initial burst release followed by a sustained release owing to swelling and increased cross-linking.
In Figure 12, sharply to 97% rises the released curcumin between 150 and 200 hours.
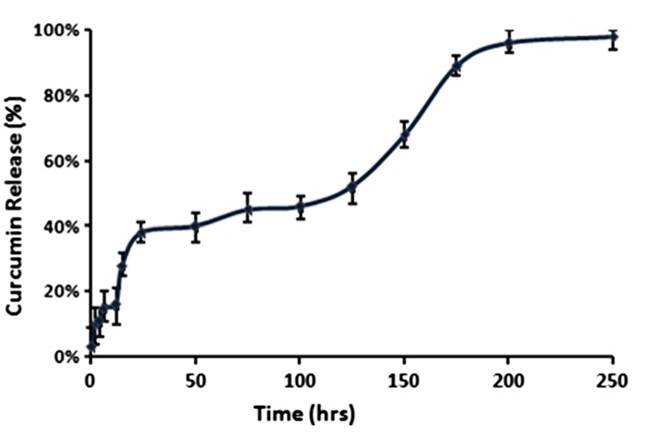
Figure 12: In vitro release curcumin encapsulated in folic acid conjugated cross-linked acrylic polymer [27]
The ability of curcumin to induce Hela cell apoptosis was evaluated graphically by fluorescence images. In Figure 13, more red dots in FA-CLAP/curcumin indicate a greater cellular uptake compared to its non-folate conjugated counterpart.
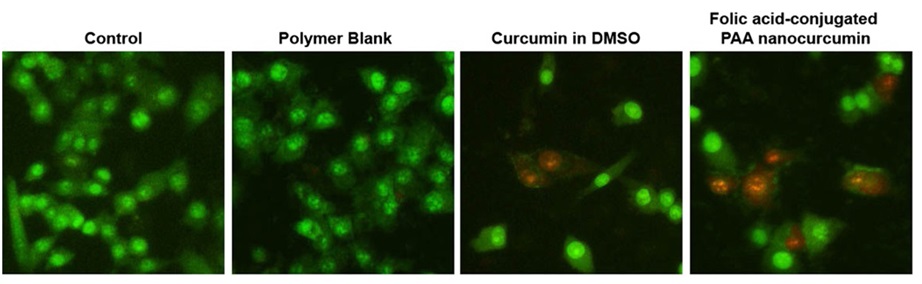
Figure 13: Fluorescence images of HeLa cells treated by polymer blank (CLAP), free curcumin, and FA-CLAP/curcumin [27]
In 2014, Varshosaz et al. employed polymeric micelles based on dextran modified with stearate and folic acid to uptake etoposide in CT-26 cells [52]. Through the utilization of both fluorescent and visible light images, it becomes evident that the inclusion of etoposide within the micelles composed of folate-grafted dextran stearate copolymer holds significant potential in enhancing the internalization of that by CT-26 cells (Figure 14).
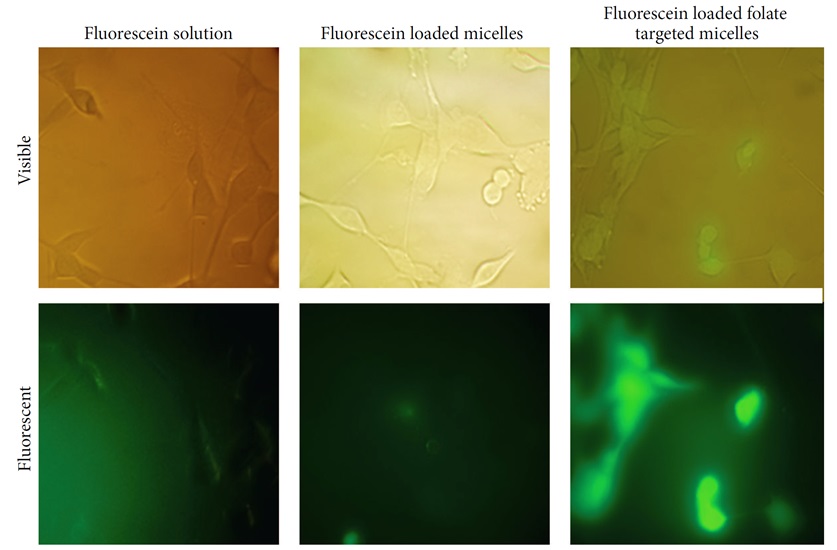
Figure 14: fluorescent and visible light images of CT-26 cells after incubation with fluorescein (fluorescent label) loaded micelles or fluorescein solution for 2 hours [37]
Lee et al. developed folic-acid-conjugated pullulan/poly(DL-lactide-co-glycolide) (FAPuLG) for the purpose of delivering doxorubicin in KB and NIH3T3 cell lines. The outcomes of in vivo revealed a notable increase in fluorescence intensity within KB solid tumors as opposed to NIH3T3 (Figure 15). These findings suggest that FAPuLG nanoparticles possess the ability to specifically target the folate receptor found in tumor cells [34].
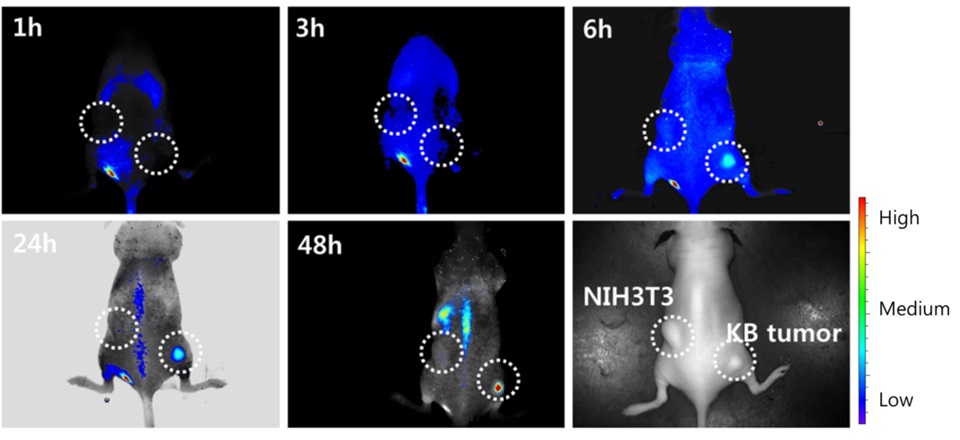
Figure 15: NIR fluorescence imaging for BALb/C nude mouse bearing an NIH3T3 and KB tumor [22]
Li et al. developed a drug delivery system utilizing poly(styrene-alt-maleic anhydride) (FA-DABA-SMA) to effectively deliver hydrophobic chemotherapeutics, such as curcumin. To evaluate the effectiveness of this delivery vehicle, PANC-1 cancer cells and RAW-Blue mouse macrophage reporter cell line were utilized, as both cell types exhibit an over-expression of folic acid receptors. Through the use of fluorescent microscopy, it was observed that both cells internalized a significant amount of curcumin when delivered with FA-DABA-SMA, in comparison to un-functionalized polymers (SMA) (Figure 16).
These findings suggest that FA-DABA-SMA polymers have the potential to serve as an active drug delivery system, targeting tumors and facilitating the internalization of hydrophobic chemotherapeutics [53].
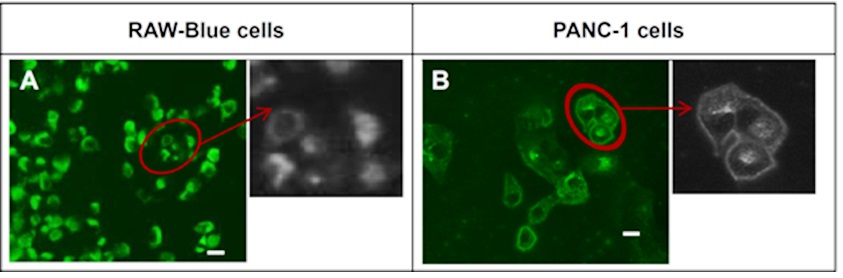
Figure 16: Fluorescent images showing cellular uptake for A) RAW-Blue cell and B) PANC-1 cell by curcumin- FA-DABA-SMA [38]
The Innovative use of carbon nanotubes@poly(N-vinylpyrrole) that has been functionalized with folic acid as a nano-platform to deliver doxorubicin was an intriguing concept that was executed by Wang et al. The resulting folic acid- nano-platform demonstrated a substantial drug-loading ratio and also showcased the ability to unload drugs in a pH-sensitive manner (Figure 17). According to Figure 17, it can be concluded that being sensitive to pH is a prominent feature in the design of this nano-platform [54].
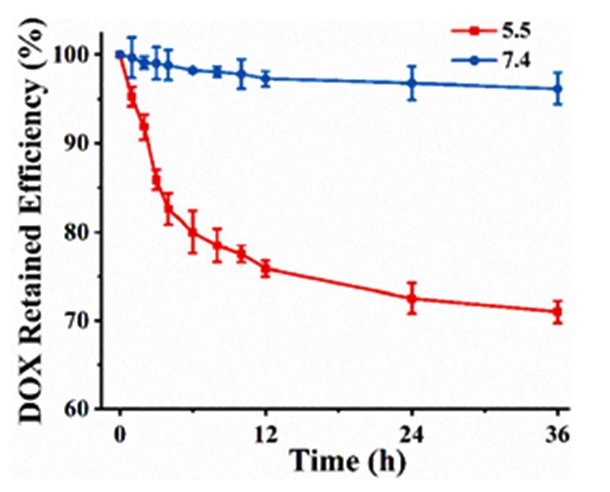
Figure 17: Controlled doxorubicin release from folic acid- carbon nanotubes@poly(N-vinylpyrrole) at pH 7.4 and 5.5 [39]
One of the properties exhibited by poly(N-vinyl pyrrole) is the enhancement of its photothermal ability. Consequently, Wang et al. integrated the techniques of chemotherapy and photothermal therapy in their investigation of HeLa cells, which were subjected to flow cytometry experiments (Figure 18). The utilization of fluorescence microscopic images in Figure 18 effectively demonstrates the influence of poly(N-vinyl pyrrole) and folic acid in cellular uptake.
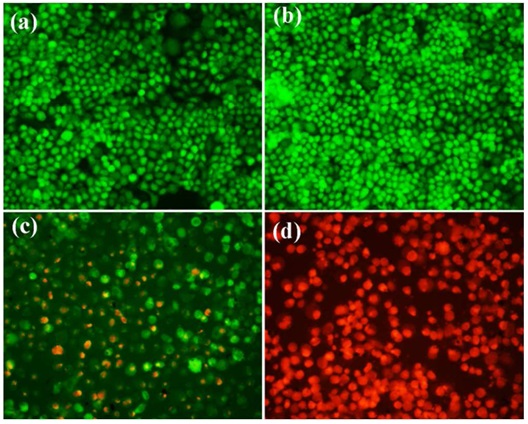
Figure 18: Fluorescence microscopic images of a) Hela cells b) HeLa cells irradiated with NIR laser c) HeLa cells incubated with folic acid-nanoplatform and d) HeLa cells incubated with folic acid-nanoplatform and NIR laser [39]
In 2017, Cheng et al. introduced the concept of synthesizing poly (ethyleneglycol)-folic acid on the surface of a polydopamine-modified mesoporous silica nanoparticle (MSNs@PDA-PEG-FA) to treat cancer through the use of dopamine monomers. The primary objective of their research was to develop efficient systems that could deliver doxorubicin to Hela cells. In Figure 19, the carrier preparation model and its delivery in the animal phase were depicted [55].

Figure 19: Graphical model of MSNs@PDA-PEG-FA delivery to animal phase [40]
Fluorescence images show that doxorubicin accumulation in tumor cells is much higher than the normal organs (Figure 20). The findings obtained from their investigations demonstrated that MSNs@PDA-PEG-FA exhibited enhanced antitumor efficacy in vivo, suggesting a highly promising potential carrier for cancer treatments.
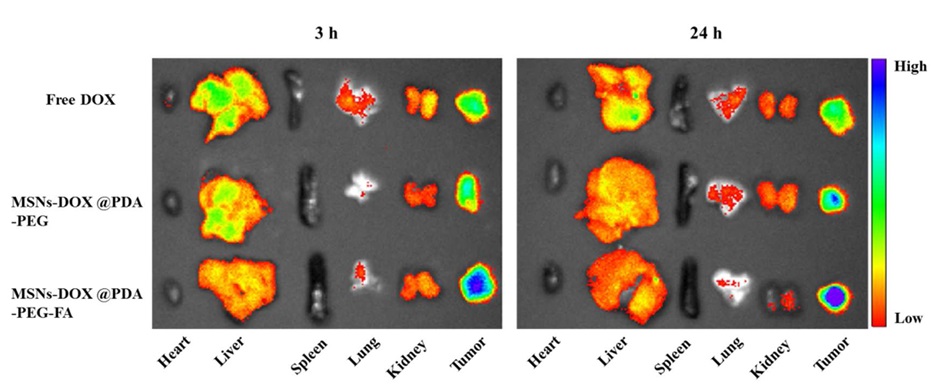
Figure 20: Ex vivo fluorescence images of major organs and tumor [40]
Zamani et al. carried out an in vivo investigation on poly(ethylene glycol)-poly (caprolactone)-functionalized folic acid (PEG-PCL-FA), which served as a pH-responsive system for the co-delivery of tamoxifen and quercetin. The images in Figure 21 show that the polymer functionalized with folic acid was the most effective type of treatment to deliver both drugs. Consequently, based on the obtained results, the researchers deduced that the polymer exhibited significant potential for the oral delivery of combination drugs, thereby making it highly applicable in clinical settings [56].
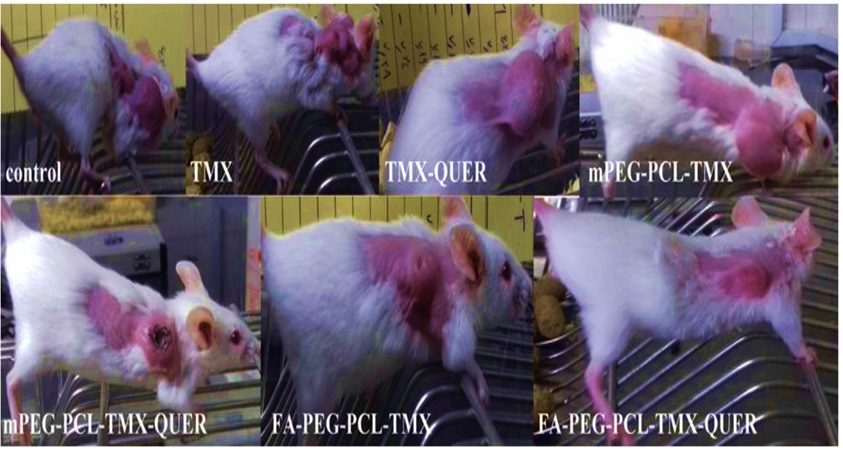
Figure 21: The schematic comparison of tumor volume after 15 days. Tamoxifen (TMX), quercetin (QUER), poly(ethylene glycol)-poly (caprolactone) (PEG-PCL) and poly(ethylene glycol)-poly (caprolactone)-functionalized folic acid (PEG-PCL-FA) [41]
Folic acid serves not only as an option of attachment to heavy polymers but also as a viable modifier for attachment to polymers with lower molecular weight. Cao et al. employed polyethyleneimine that had been modified with folic acid to achieve targeted delivery of pDNA [57]. The findings derived from flow cytometry revealed that the facilitated uptake of the synthesized polymer into specific cells via the folate receptor also played a pivotal role in its exceptional efficiency in gene delivery. The presence of red dots in the cell nuclei indicates significant distribution of pDNA using the functionalized polymer (Figure 22).
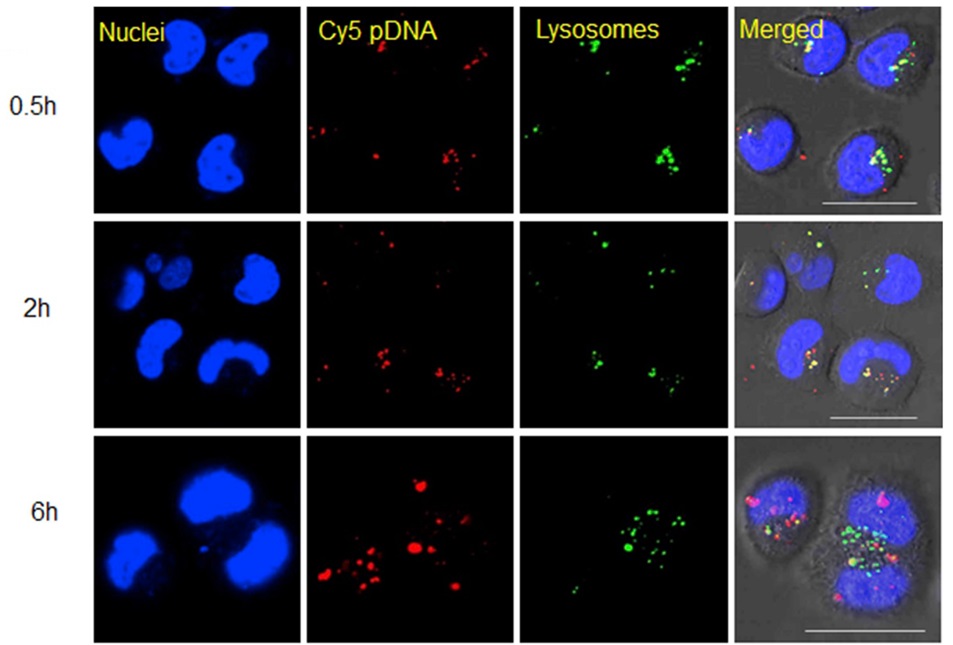
Figure 22: Subcellular distribution of the folic acid- polyethyleneimine/pDNA in HeLa cells [42]
Yang et al. developed a folic acid-decorated star polymer consisting of β-cyclodextrin-based poly(ε-caprolactone)/dextran. Figure 23 depicts the graphical model of the formation of theranostic polymer nanoparticles and intracellular distribution [58].
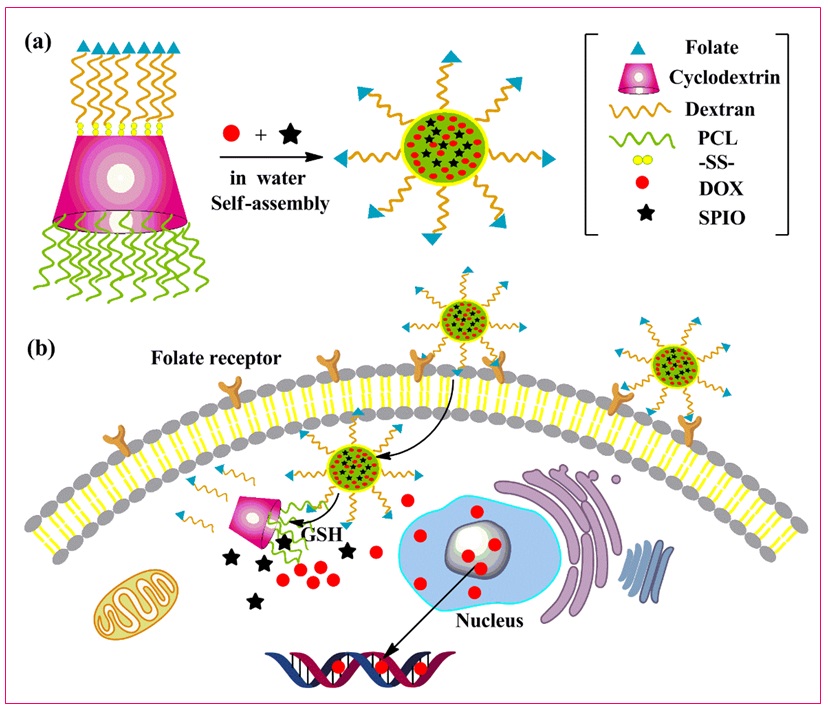
Figure 23: Graphical model for a) the formation of theranostic polymer nanoparticles and b) intracellular distribution of decorated polymer. poly(ε-caprolactone) (PCL), disulfide bond-linked (SS), doxorubicin (DOX), superparamagnetic iron oxide particles (SPIO) [43]
The final polymer can be utilized in the fields of chemotherapy and MRI. The researchers loaded doxorubicin and iron oxide particles into the polymer nanoparticles for MRI detection and delivery to HepG2 cells. It is crystal clear that as the concentration of iron in the final polymer increases, the signals darken dramatically, indicating an improved relaxation property in MRI (Figure 24).
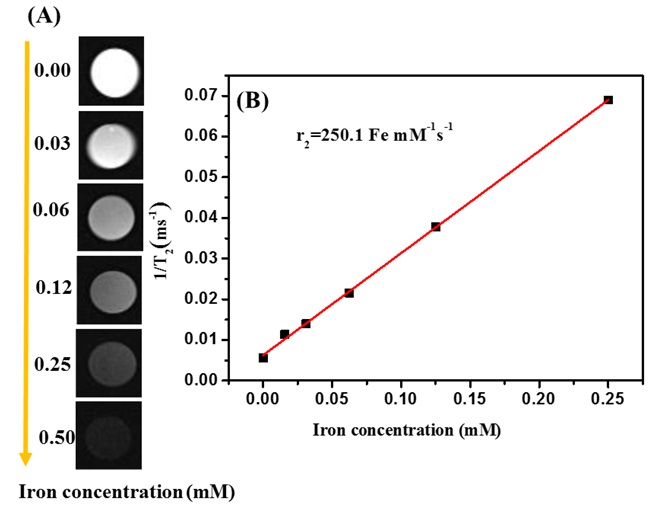
Figure 24: A) T2-mapping images of decorated nanoparticles at various Fe concentrations (mM) and B) T2 relaxation rate [43]
In 2021, Zhao et al. designed a folic acid-based poly(ethylene glycol) to facilitate the delivery of paclitaxel nanocrystals (NC@lipid−PEG−FA) to treat breast cancer [59]. Studies regarding tissue distribution and the impact on tumor growth in breast cancer-bearing nude mice demonstrated that paclitaxel-NC@lipid−PEG−FA considerably augmented the accumulation of paclitaxel within the tumor site and effectively hindered the progression of the tumor, in comparison to paclitaxel-NC@lipid−PEG, paclitaxel-NC, or free paclitaxel (Figure 25).
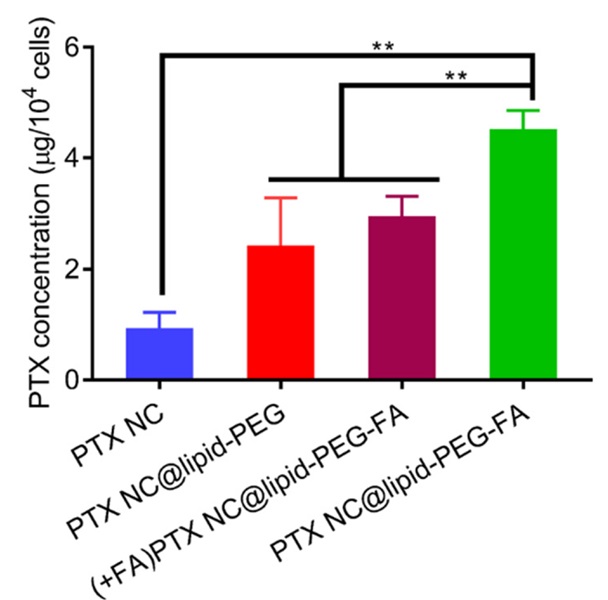
Figure 25: Tumor weight exposed with various delivery formulations [44]
Most of the research conducted thus far has primarily focused on investigating the functionalization of polymers utilizing folic acid, with the main objective being the treatment of cancer [60-62]. However, an intriguing study emerged that explored the application of conjugated polymers in the context of combating rheumatoid arthritis. Zhang et al. effectively employed folic acid-glucosamine/methotrexate (FA-Glu/MTX) as an option to assess the stability, sustained release properties, cytotoxicity, and therapeutic impact for the treatment of rheumatoid arthritis. In Figure 26, the mechanism of action of FA-Glu/MTX is illustrated [63].
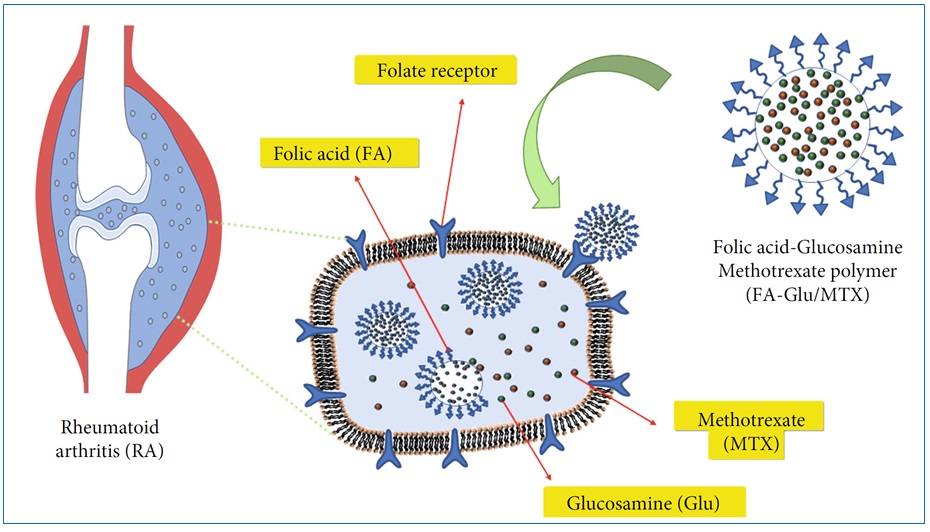
Figure 26: Diagram of the mechanism of action of FA-Glu/MTX on rheumatoid arthritis [45]
In animal studies with the degree of foot swelling, it was found that FA-Glu/MTX had a good performance in the treatment of rheumatoid arthritis (Figure 27).
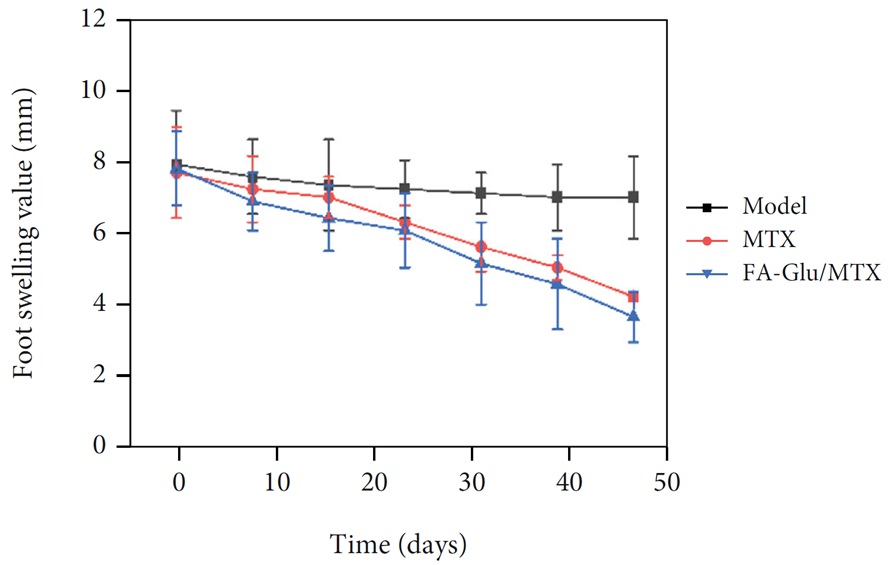
Figure 27: Comparative analysis of the effects of FA-Glu/MTX and methotrexate (MTX) on the degree of swelling of the rat’s foot [45]
Recognition of Folic Acid by Folate Receptors
The preceding sections have presented the findings of scientific investigations into the targeted delivery of drugs and the modification of polymers through folic acid. It is worth noting that a crucial aspect is the comprehension of the folate acceptor's structure, which can ultimately facilitate the appropriate development of carrier polymers. Folate receptors, namely FRα, FRβ, and FRγ, are glycoproteins located on the surface of cells that are rich in cysteine. These receptors possess a strong affinity for folate, enabling them to facilitate the cellular uptake of folate. While they are expressed at significantly low levels in most tissues, folate receptors, particularly FRα, are highly expressed in numerous cancer cells to fulfill the folate requirements of rapidly dividing cells in conditions where folate is scarce [25,64].
Chen et al. conducted an interesting study to understand how folic acid interacts with its receptors [65]. FRα exhibits a globular structure stabilized by eight disulfide bonds and contains a deep open folate-binding pocket. The fundamental mechanism underlying the affinity between folic acid and FRα is predicated upon the establishment of hydrogen bonds and hydrophobic interactions (Figure 28).
In Figure 29, the interaction map of folic acid with FRα is presented. Generally, carboxylic acid group 2 (Scheme 1) manifests as an appropriate moiety for bonding with polymers and diverse pharmaceutical agents. This is due to the unhindered state of its hydroxyl group, which does not engage with acceptor amino acids. N-H groups play a crucial role in forming bonds with FRα and, whenever possible, should avoid bonding with polymers.
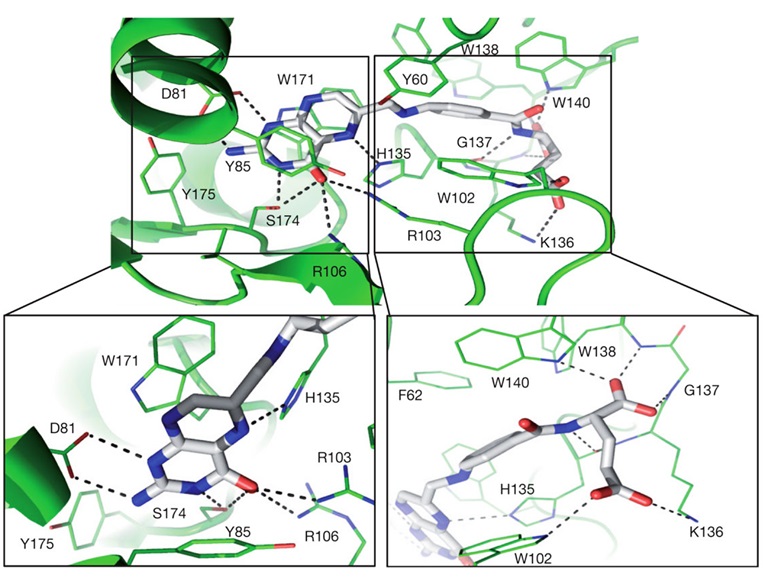
Figure 28: Folic acid-binding network with close-ups of the folic acid head and tail groups of FRα
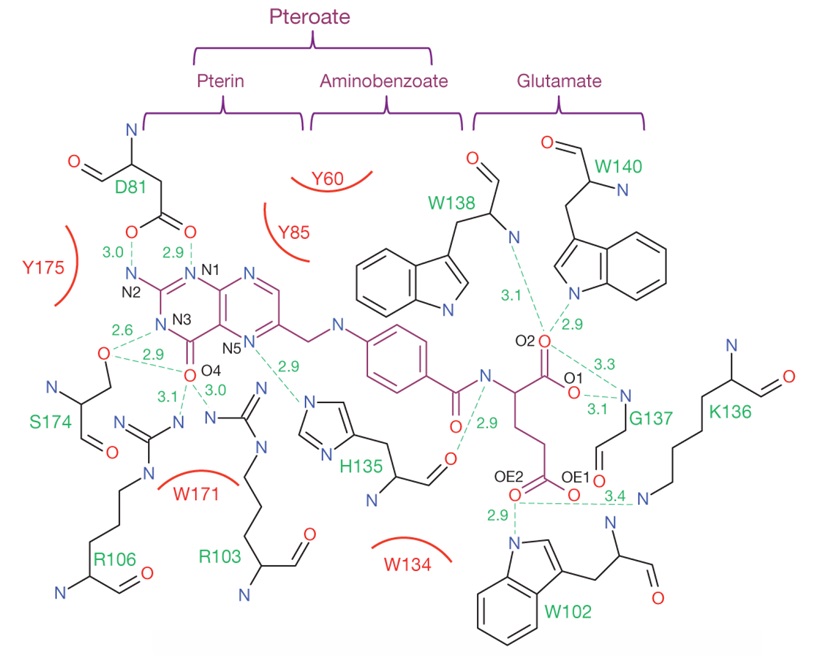
Figure 29: Interaction map of folic acid with ligand-binding-pocket residues of FRα
If the intention is to derive a general conclusion from the research conducted in the domain of polymer-based targeted drug delivery incorporating folic acid and its interaction with folate receptors, it can be stated that this particular matter can be examined from two distinct perspectives. The initial aspect encompasses the proficient synthesis of targeted drug delivery systems utilizing folic acid, while the subsequent aspect pertains to the examination of the conduct of folic acid during cellular entry, which can be explored within the realm of the natural endocytosis pathway.
Folic acid, indeed, employs the endocytosis pathway as a molecule that undergoes internalization via the membrane, thereby guiding the polymer system into the cellular milieu. Consequently, folic acid is recognized as an exploiter of the endocytosis pathways in the process of targeted drug delivery.
Conclusion
Today, the field of targeted drug delivery has entered a new phase, characterized by significant advancements that are anticipated to have a profound impact in the near future. The incorporation of specific molecules, such as folic acid, in the drug delivery process has yielded advantageous outcomes. Folic acid, with its active functional groups, particularly the carboxylic acid groups, can be chemically bonded to a wide range of polymers using appropriate synthetic methods. Through an examination of folic acid conjugated with polymers, it becomes evident that the intracellular distribution of the loaded drug is enhanced, leading to a proper release of the drug and subsequently resulting in notable toxic effects. These claims are substantiated by both in vivo and in vitro studies. An investigation into the interaction between folic acid and its acceptors elucidated the role of N-H groups in the formation of hydrogen bonds. Ultimately, the use of folic acid in polymer modification proves to be efficacious in cases where the target cell possesses a folate acceptor.
Acknowledgments
Financial support of this study was given by Research and Technology Vice-chancellor of Hamadan University of Medical Sciences with grant No. IR.UMSHA.REC.1400.574 related to Taghvaei's thesis.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed toward data analysis, drafting, and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of Interest
The authors declare that they have no conflicts of interest in this article.
ORCID
Asrin Bahmani
https://orcid.org/0000-0002-0258-1027
Gholamabbas Chehardoli
https://orcid.org/0000-0002-8760-3837
HOW TO CITE THIS ARTICLE
Asrin Bahmani, Alireza Taghvaei, Farzin Firozian, Gholamabbas Chehardoli. Folic Acid as an Exploiter of Natural Endocytosis Pathways in Drug Delivery. Chem. Methodol., 2024, 8(2) 96-122.
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER