Document Type : Original Article
Authors
- Zahra Sabouri 1
- Shirin Sammak 1
- Sajjad Sabouri 2
- Samaneh Sadat Tabrizi Hafez Moghaddas 1
- Majid Darroudi 3
1 Department of Medical Biotechnology and Nanotechnology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2 Natural Disasters Prevention Research Center, School of Civil Engineering, Iran University of Science and Technology, Tehran, Iran
3 Department of Basic Medical Sciences, Neyshabur University of Medical Sciences, Neyshabur, Iran
Abstract
This study was conducted to evaluate the photocatalytic applications and the cytotoxicity effects of Ag-Se doped ZnO-Co3O4-NiO fivenary nanocomposite synthesized using polyanionic cellulose (PAC) polymer as a stabilizer agent. Several procedures such as XRD, FESEM, FTIR, EDX, PSA, and UV-Vis were applied to investigate synthesized nanocomposite. Consistent with the FTIR spectrum, chemical bonds were seen in the structure of the nanocomposite which approved the successful synthesis of them. The XRD pattern of the synthesized nanocomposite revealed sharp diffraction peaks with high crystallinity, and pure phases of Se, Ag, ZnO, NiO, and Co3O4 were approved with XRD analysis. FESEM/PSA images indicate that nanocomposite was synthesized with an average size of 23-49 nm and relatively uniformly distributed, in addition, it has a spherical morphology. The synthesized nanocomposite exhibited excellent photocatalytic activity to methyl orange (MO) dye degradation under a UVA light source. The degradation rate of nanocomposite reached 99% within 80 min. The kinetic studies indicate that the degradation of MO dye follows a first-order kinetic model. The cytotoxicity of the nanomaterial was assessed on normal mouse fibroblast NIH3T3 and cancer mouse melanoma B16F0 cell lines with the MTT assay. The results of the MTT test revealed significant cytotoxic influences on cancer B16F0 cells (IC50 value = 258.5 µg/mL) in comparison to normal cells.
Graphical Abstract
Keywords
Main Subjects
Introduction
Industrial effluents and wastewater generated from organic dye usage are typically toxic to aquatic organisms and humans [1]. Therefore, the efficient and environmentally friendly removal of dyes from wastewater is of utmost importance for clean production practices. Conventional treatment methods for organic dye wastewater are expensive, time-consuming, prone to secondary pollution, and often result in incomplete degradation [2]. In this regard, photocatalytic technology offers a promising solution for the more effective degradation of dye wastewater. Among the various photocatalysts, metal oxides have garnered attention due to their desirable properties such as non-toxicity, chemical inertness, and durability [3-5]. To enhance the photocatalytic activity of metal oxides, several methods have been extensively investigated, including compounding, noble metal deposition, and doping with metal and non-metal ions. Metal oxides (MOs) play an important role in various fields of science, such as chemistry, physics, medicine, and engineering [6,7]. MOs with nanostructured morphologies are widely studied due to their remarkable optical [8], chemical [9], and photocatalytic properties [10-12]. It is also known that these properties depend on both the morphology and the size of the nanoparticles. However, the processes for controlling particle morphology and size are often complex and expensive [13]. Morphological diversity is one of the remarkable features of NCPs which formed 0 to 3D nanoparticles [14], nanowires [15], nanofibers [16], nanorods [17,18], and nanosheets [19]. It was found that 1-D structures are excellent for gas sensing applications due to their high surface-to-volume ratio [20], while 2-D structures, along with gas sensing devices, are more suitable for constructing supercapacitors and other devices [21]. Although pure nanocomposites have numerous applications, doping them with different metals can improve their properties and make them suitable for many other potential applications [22]. As is well-known, doping is the process of introducing impurities into a semiconductor to modify its conductivity or properties. It was found that doping of nanocomposite can be performed with different elements such as Se and Ag. Se is a practical method for modifying its optical properties and Silver (Ag) is another metal that could be used for doping nanocomposite due to its high solubility and large ion size [22]. In addition, Ag and Se ions can act as acceptors when doped with ZnO-Co3O4-NiO nanocomposite due to their excellent properties and potential for easy substitution.
In this study, an eco-friendly route was presented for the synthesis of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite using PAC polymer as a stabilizing agent [23]. This method has some advantages including simplicity, environmental compatibility, low-cost, high-purity precursors, and the production of minimal by-products. Moreover, the Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite can be prepared at low temperatures without the use of harmful chemicals [24]. Although research has been conducted on the synthesis method and photocatalytic effect of nanocomposites, there is no report on the use of PAC polymer as a reducing agent for the synthesis of nanocomposite [7].
After synthesis, the Ag-Se doped ZnO-Co3O4-NiO fivenary nanocomposite was characterized with FT-IR, FESEM, XRD, PSA, EDX, and UV-Vis analysis. In continuation, the nanocomposite cytotoxicity was evaluated on normal mouse fibroblast NIH3T3 and cancer mouse melanoma B16F0 cell lines usage of the MTT assay. In addition, the synthesized nanocomposite was used as a photocatalyst in methyl orange (MO) degradation under UVA irradiation. So far, no other study has been done on the biosynthesis of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite via PAC polymer, which can express the innovation and originality of this study.
Experimental
Reagents
Polyanionic cellulose (PAC), Sodium hydroxide (NaOH, 98%, Merck), Ni(NO3)2. 6H2O, Zn(NO3)2. 6H2O, Co(NO3)2. 6H2O, Na2SeO3, AgNO3 (≥ 99%), and methyl orange (C14H14N3NaO3S) were bought from Sigma or Merck companies. All chemicals were used without further purification. The normal mouse fibroblast NIH3T3 and cancer mouse melanoma B16F0 lines were prepared of the Pasteur Institute, Iran.
Preparation of Ag/Se Doped Zno-Co3O4-Nio Fivenary Nanocomposite
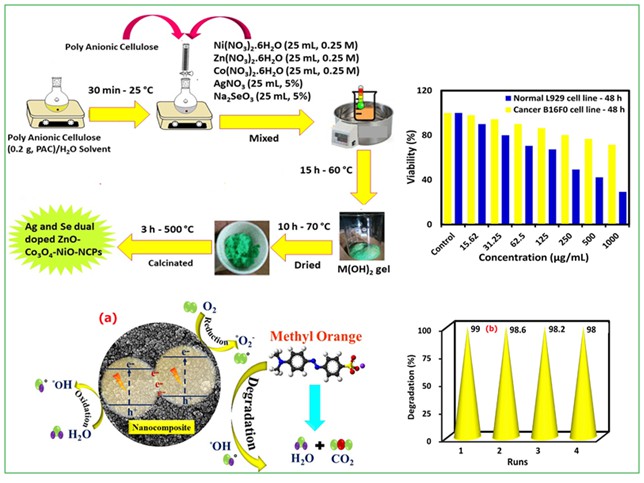
In this study, the Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite was synthesized using the eco-friendly method. Initial, solutions of Ni(NO3)2. 6H2O (25 mL, 0.25 M), Zn(NO3)2. 6H2O (25 mL, 0.25 M), and Co(NO3)2. 6H2O (25 mL, 0.25 M) salts were prepared separately, and then the solution of the salts was mixed and stirred at room temperature for 15 min (A). Afterwards, the prepared solutions of AgNO3 (25 mL, 5%) and Na2SeO3 (25 mL, 5%) were added to A solution and stirred for another 15 min (B). Thereafter, 0.2 g of polyanionic cellulose (PAC) polymer was dissolved in deionized water to obtain the PAC solution (C). Next, 30 mL C solution was added to the B solution and the resulting mixture was stirred at 60 °C for 15 h. The collected gel was placed in the oven at 70 °C (10 h) and then calcinated at 500 °C (3 h) to achieve Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite [25,26]. The preparation plan of the nanomaterial is exhibited in Figure 1.
Characterization
The characterization of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite was done with numerous studies. The chemical bonds presence in the synthesized sample was approved via FTIR analysis. The optical properties of nanomaterials were examined using UV-Vis analysis. FESEM/EDX device was employed to assess the morphology of the synthesized nanomaterial. The determination of the phase and crystal structure of nanomaterial was assessed using the XRD pattern.
Statistical Analysis
The Prism® software and ANOVA test were used for the investigation results of the MTT studies. The p-value ≤ 0.05 was considered significant. Also, Image J® software and IBM SPSS 22® software were applied to the dragging of particle size distribution curves
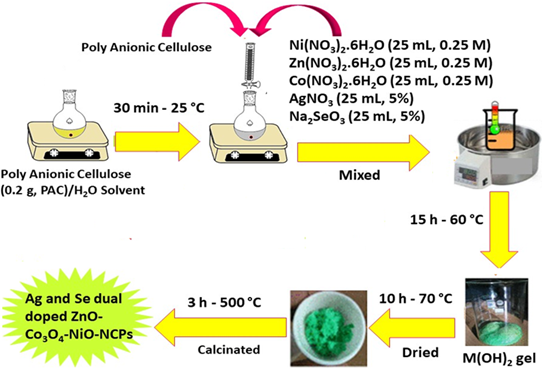
Figure1: Synthesis plan of the nanocomposite
Cell section
Culture of Cell
After DMEM media preparation with FBS (10%)/antibiotic (1%), normal NIH3T3 and cancer B16F0 cell lines were posited for 24 h on the 96-well plate to stick to the bottom of the well under incubation conditions of 37 C and 5% CO2.
Cytotoxicity
The cytotoxicity of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite was assessed on the normal mouse fibroblast NIH3T3 and cancer mouse melanoma B16F0 cell lines through MTT assay [27,28]. Cells were initally posited on the 96-well plate (7×103 cells in each well) to stick to the bottom of each well under incubation conditions of 37 C and 5% CO2 for 24 h. Next, the cells were treated with several concentrations of nanomaterial (0 and 1000 μg/mL) for 48 h. In the continuing, the MTT solution (50 uL, 5.0 mg/mL) was added to every well and incubated for 4 h. Afterwards, 100 µL of dimethyl sulfoxide (DMSO) was appended to each well and shaken for 15 min to dissolve formazan crystals. In the final, the absorbance was measured at 630 nm and the cell survival was calculated usage of Equation (1). The cytotoxicity tests were performed in triplicate.
![]()
Where indicates the treated sample and denotes the control group. The outcomes of the MTT assay (Figure 2) showed dose-dependent toxicity of nanocomposite on cancer B16F0 cells contrasted to normal NIH3T3 cells. The IC50 value showed that nanocomposite can inhibit cancer cells after 48 h in a concentration of 258.5 μg/mL. Also, outcomes revealed that synthesized nanomaterials can prevent the growth of cancer cells in a dose-dependent behavior, but no inhibition effects were observed on normal NIH3T3 cells.
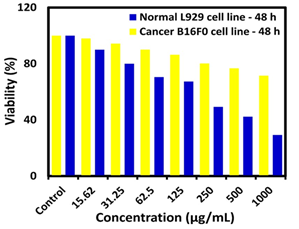
Figure 2: Result of the MTT assay on mouse fibroblast NIH3T3 and mouse melanoma B16F0 cell lines after 48 h, P-value <0.05 is considered significant (n = 3)
Photocatalytic Application
Assessment of Photodegradation
According to the details designated elsewhere, an MO photodegradation test was conducted [29,30]. The photocatalytic degradation of the nanocomposite was examined for 80 min, and the outcomes are presented in Figure 3a.
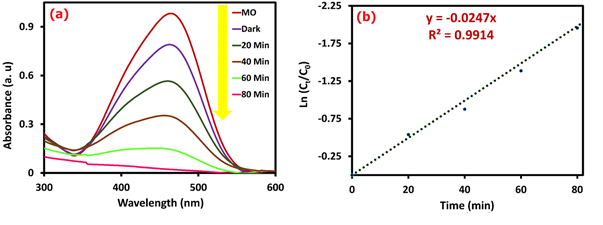
Figure 3: UV-Vis results for photocatalytic test (a) and kinetic chart for MO photodegradation (b)
The photocatalytic degradation test was carried out using a hand-made photocatalytic reaction device to investigate the photocatalytic activity of nanocomposite to the degradation of MO pollutant under UV light (20 W, 365 nm) irradiation. The synthesized photocatalyst was added to 50 mL of MO solution with a concentration of 10 mg/L (pH = 9). A blank control was also prepared using the same concentration and volume of MO but without the photocatalyst. The photocatalyst and the MO solution were mixed well, and then the mixture was placed in a dark treatment for 40 min to establish an adsorption equilibrium between the photocatalyst and MO solution. Afterwards, the UV light source was turned on, and the 2 mL suspension of the mixture was centrifuged every 20 min. The absorbance was measured employing a UV-Vis device at the wavelength of 464 nm. The degradation percentage of the MO was computed with Equation 2, which was offered about 99% after 80 min.
![]()
Where, indicates time t sample and denotes the pre-radiation sample. It was observed that nanocomposite exhibited significant photocatalytic degradation performance. These findings highlight the high photocatalytic activity of nanocomposite, indicating the useful effect of Ag and Se doping in easing the degradation of MO dye. Based on previous reports, the degradation process of MO dye by the photocatalyst follows the primary reaction kinetic model (Equation 3).

Where, C0 point is the initial concentration of MO dye, Ct is the residual concentration of MO (mg/L) after t min of UV irradiation, and k is the reaction rate constant (min-1). The catalyst's activity can be investigated based on the amount of rate constant (Kobs). The findings (Figure 3b) showed that the nanocomposite follows the first-order kinetic model with a Kobs value equal to 0.0247 min-1.
According to the mechanism of photocatalytic activity (Figure 4 (a)), it is known that with UV light irradiation to the catalyst surface, a large number of superoxide and hydroxyl radicals ( and OH°) will be generated due to the catalytic effect of the nanocomposite. The and OH° radicals have strong oxidizing properties, so they will react with MO dye to oxidize it to decompose into CO2, H2O, etc. The hydroxyl radicals and superoxide radicals will continue to degrade, until turning the MO dye into non-harmful ingredient combinations. The reactions of MO dye photodegradation are exhibited below (Reactions 1 to 5).
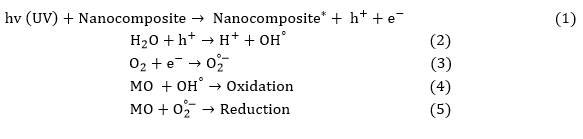
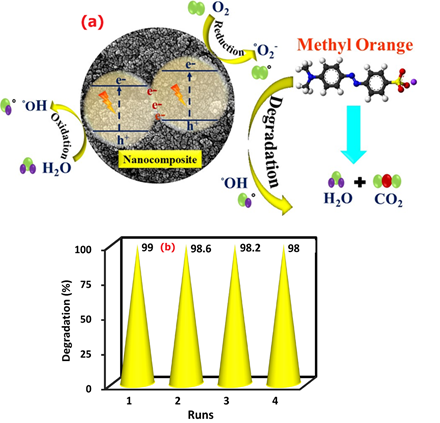
Figure 4: Photodegradation mechanism of methyl orange dye (a) and recovery of nanocomposite (b)
Moreover, the reusability of a catalyst plays an important role in its experimental applications. After a thorough examination of their photocatalytic performance, the Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite underwent separation via centrifugation (10 min, 10,000 rpm), followed by a washing process involving distilled water and ultimately, drying at 70 °C for 12 h. As evidenced by Figure 4(b), the resultant nanocatalyst was utilized on multiple occasions, with a slight reduction in degradation percentage observed due to the solubility of nanocomposite in the water solvent [35,36].
Results and Discussion
XRD
As demonstrated in Figure 5, the XRD pattern of the Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite confirmed pure phases of Se, Ag, ZnO, NiO, and Co3O4 with crystallite structures. The XRD pattern reveals sharp diffraction peaks, indicating the high crystallinity of the sample. No additional peaks were observed in the calcined sample, which indicates the high purity of the product. The detected patterns match well with the standard card #JCPDS#36-145, approving the hexagonal structure of ZnO. These diffraction peaks appear at 2θ of 31.96, 34.65, 36.44, 47.70, 56.73, 63.09, 66.53, 68.26, and 69.39°, which matching the crystallographic planes of (100), (002), (101), (102), (110), (103), (200), (112), and (201), respectively [31,32]. The XRD peaks, detected at 2θ: 36.93, 42.82, 62.6, 75.3, and 79.2◦, were related via (111), (200), (220), (311), and (222) crystal planes that cubic crystalline structure (FCC) of NiO. The XRD peaks detected at 2θ: 19.3°, 31.2°, 36.8°, 44.8°, 59.3°, and 65.2° are correlated to the (111), (220), (311), (400), (511), and (440) crystal planes which confirmed face-centered cubic (FCC) structure of Co3O4.

Figure 5: XRD of the nanocomposite
The XRD peaks revealed the cubic structure of Ag at 2θ: 38.25°, 44.41°, 64.55°, and 77.60° which are in accordance via (111), (200), (220), and (311) planes [33,34]. The peaks observed at 2θ: 23.02°, 31.49°, 44.40°, 45.80°, and 56.0° correspond to (100), (101), (012), and (110) planes that are indicative of the hexagonal structure of Se [35,36]. The size of the nanocomposite was computed through Equation 4, which was about 33 nm.

FTIR
To examine the chemical structure and type of bonds, the FT-IR spectrum of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite was presented in the range of 400 to 4000 cm-1 (Figure 6(a)). According to the comparison table of functional groups in the infrared spectrum, the range of 3351 to 3528 cm-1 shows wide absorption peaks, which may be owing to the free and bonded hydroxyl groups. The O-H stretching vibrations of the H2O molecules were seen as a strong band at 3426 cm-1. The observed band at 2354 cm-1 is due to CO2 molecules in the air. The strong absorption peak exists at 1621 cm-1, which may be the characteristic peak of O-H bending vibrations of the water molecules. Furthermore, the bands that were seen in the range 400 and 1000 cm-1 can be attributed to the stretching vibrations of the metal-oxygen (M-O) bonds. Therefore, based on the infrared absorption spectrum, it is found that nanocomposite was successfully synthesized [25,26].
UV-Vis/Bandgap
UV-Vis spectra of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite are presented in Figure 6(b). The absorption band that appeared at the wavelength of 316 nm is attributed to ligand-to-metal charge transfers (LMCT). The absorption bands in UV areas could also be owing to the electron move of the valence band to the conduction band. As a consequence, electron-hole recombination reduces, and oxidizing radical creation rises which leads to increasing photoreaction performance. Using the data of the UV-Vis spectrum, the graph of in terms of drawn, and the band gap energy was obtained from the point of intersection of the tangent line on the curve with the horizontal axis.
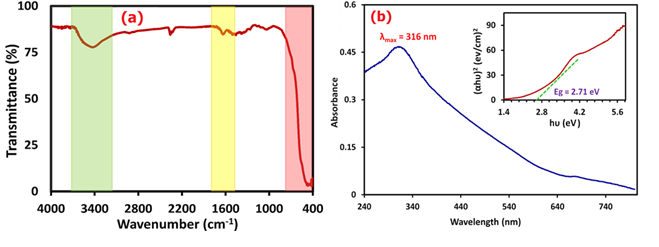
Figure 6: FTIR analysis (a) and UV-Vis/Bandgap spectra (b) of the nanocomposite
Equation (5) was used to obtain the band gap energy of the nanocomposite. The band gap diagram of the nanocomposite is presented in Figure 6(b). The amount of band gap energy calculated was equal to 2.71 eV [37,38].
![]()
FESEM/EDX/PSA
The morphology, size, and elements distribution of Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite was investigated through FESEM/EDX/PSA images and the results are presented in Figure 7(a-g).
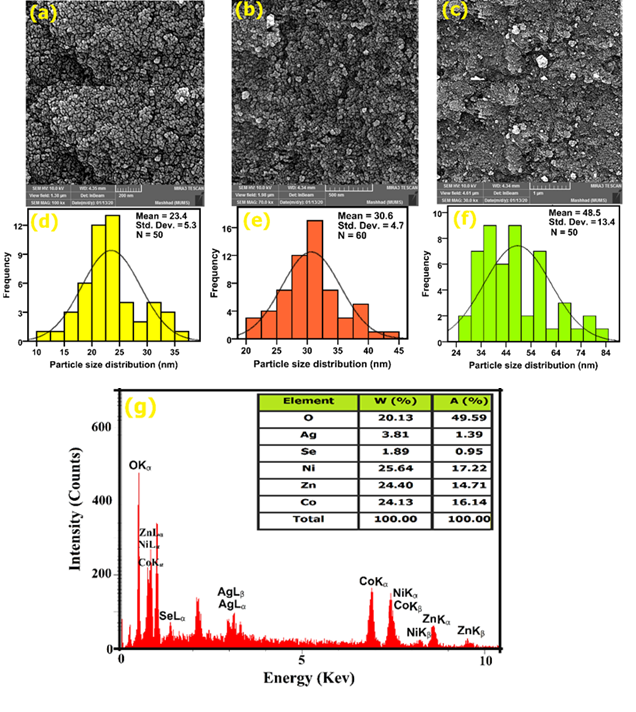
Figure 7: FESEM images (a–c), PSA curves (d–f), and EDX analysis (g) of the nanocomposite
The results show that the nanocomposite was synthesized with high purity. FESEM images (Figure 7(a-c)), indicate that nanocomposite was synthesized with small particle size, relatively uniformly distributed, and completely developed crystals, and also has a spherical morphology. The size average of the nanocomposite was found to be about 23 to 48.5 nm using the PSA curves (Figure 7(d-f)). The particle size distribution is consistent with the obtained results of the XRD pattern. To know the quantity and nature of the elements used, as well as the amount of element dispersion in the synthesized sample, the nanocomposite was analyzed by the EDX technique. As shown in Figure 7(g), clear six elements of O, Se, Ag, Ni, Co, and Zn were detected in the synthesized sample, and the weight and atomic percentages of the elements were inserted in Figure 7(g) [39,40].
Conclusion
In this study, Ag/Se doped ZnO-Co3O4-NiO fivenary nanocomposite was prepared via polyanionic cellulose (PAC) polymer with the green chemistry method. Different analyses showed that the synthesized nanocomposite has good uniformity and dispersion. The FESEM images showed that these particles have a spherical morphology with uniformity distribution. The cytotoxicity of the nanocomposite was assessed on NIH3T3 and B16F0 cell lines with the MTT test. The results revealed significant cytotoxic influences on cancer B16F0 cells (IC50 = 258.5 µg/mL) in comparison to normal NIH3T3 cells. The synthesized nanocomposite exhibited excellent photocatalytic activity to MO dye degradation under a UVA light source and the degradation rate reached 99% within 80 min. The experimental results indicate that the degradation of MO dye follows a first-order kinetic model. This study provides a valuable reference and offers solutions to enhance the practical application of photocatalytic materials for wastewater degradation. Concerning the high efficiency of the photocatalytic process, we recommend utilizing this method for the removal of pollutants in water and wastewater treatment processes.
Funding
This project was financially supported by the Vice-Chancellor for Research (Grant no. 4011401), Mashhad University of Medical Sciences.
Conflict of Interest
The authors declare that they have no conflict of interest in this study.
Ethical Approval
No experiments are conducted on animals or humans.
Availability of Data and Material
Not applicable.
ORCID
Majid Darroudi
https://orcid.org/0000-0002-2624-7242
HOW TO CITE THIS ARTICLE
Zahra Sabouri, Shirin Sammak, Sajjad Sabouri, Samaneh Sadat Tabrizi Hafez Moghaddas, Majid Darroudi. Green Synthesis of Ag-Se doped ZnO-Co3O4-NiO fivenary Nanocomposite using Poly Anionic Cellulose and Evaluation of Their Anticancer and Photocatalyst Applications. Chem. Methodol., 2024, 8(3) 164-176
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company