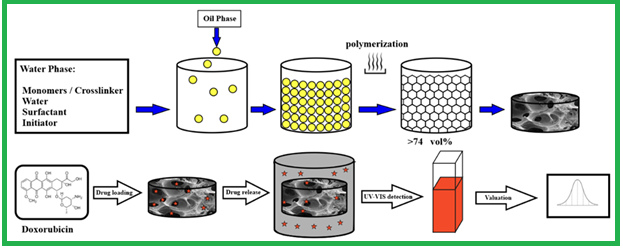
Document Type : Original Article
Authors
Department of Chemistry, Payame Noor University, 19395-4697 Tehran, Iran
Abstract
In recent years, biomedicine has focused extensively on developing a biologically versatile drug delivery system characterized by responsive behavior and customizable properties. Among drug carriers, hydrogels can be a suitable option. Since they have specific surface and structure to selectively maintain and transport the drug to the operation area, they are released in a favorable time frame to provide a higher therapeutic effect. Here, we announce the synthesis of co-polymer of poly (sodium alginate (Alg) and 2-hydroxyethyl methacrylate (HEMA)) in high internal phase emulsions (HIPEs), to produce highly porous hydrogel which has been developed to load chemotherapeutic drug doxorubicin (DOX). The percent of porosity can be changed with the variables involved in the polymer synthesis procedure. The developed beads were characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and scanning electron microscopy (SEM). In vitro release studies were investigated in pH 5.4 and 7.4 at 37 and 42 °C, it was shown that DOX was effectively incorporated into the porous hydrogel and released in a controlled manner through pH regulation and the swelling-shrinking process. The presence of hydroxyl and carboxylic acid groups in the structure of synthesized poly HIPE enhances the pH-sensitivity and swelling behavior of the resulted hydrogel, which can be designed to release drugs selectively in response to the acidic conditions of the tumour, offering a promising strategy for localized and effective cancer therapy.
Graphical Abstract
Keywords
Main Subjects
Introduction
Polymerized High Internal Phase Emulsion (Poly HIPEs) represents porous polymers synthesized within the continuous external phases of high internal phase emulsions (HIPEs). The predominant portion of this emulsion is the dispersed phase (the internal phase), constituting a volume fraction of at least 0.74 [1-5]. Through the removal of the internal phase and the polymerization of the external phase, specialized voids are created. These voids are interconnected through secondary pores or channels known as "throats", resulting in an open-porous and highly interconnected structure. Both voids and throats are categorized as macro pores (ranging from 1 to 100 micrometers), with a density typically below 0.1 g/cm3 [6]. The advantageous combination of low density and high porosity positions polyHIPEs as a favorable alternative for various applications, including gas storage [7,8], catalysis [9-10], chromatography [11,12], absorption [13-17], and tissue engineering [18,19]. The most studied poly HIPE materials are based on hydrophobic monomers such as styrene and divinyl benzene synthesized in water in an oil W/O emulsion [20,21]. Recently, special attention has been paid to the synthesis of polymers with water-soluble monomers, because they are widely used in biomedical application, tissue engineering, or drug delivery systems [22-24]. Such delivery frameworks offer various benefits compared to conventional dosage structures, including improved efficiency, achieving prolonged or controlled drug release, enhancing bioavailability or stability, and facilitating targeted drug delivery to specific sites [25-27]. Similarly, beds also offer advantages such as minimizing fluctuations within the therapeutic range, reducing side effects, decreasing dose frequency, and promoting consistent therapeutic efficacy. Concerning the abundance and biocompatibility, natural polymers like polysaccharides have been utilized in drug-delivery applications [28-31]. One such polymer is the biopolymer alginate, derived primarily from brown algae of the phaeophyceae class and composed of two monomeric units, β-D-mannuronic acid and α-L-guluronic acid [32]. Alginates exhibit significant potential as drug delivery carriers and frameworks for tissue engineering due to their versatile properties, including biocompatibility, hydrophilicity, biodegradability, non-toxicity, and considerable promise in drug delivery applications [33]. Nevertheless, their innate disadvantages, such as inadequate mechanical strength, uncontrolled degradation, and extensive water absorption properties, lead to unpredictable and uncontrolled release rates of the active ingredients. To overcome these challenges and formulate more successful products compared to those exclusively utilizing sodium alginate, a viable solution involves the combination of hydroxyethyl methacrylate (HEMA) and sodium alginate through grafting. Poly (HEMA) (PHEMA) exhibits swelling behavior but remains insoluble in water, allowing it to retain water within its structure. PHEMA possesses excellent biocompatibility and mechanical strength, making it well-suited for biomedical applications such as drug delivery. In addition, its hydrogels have demonstrated significant potential for implantable applications, including bio-hybrid artificial organs, and are widely utilized in commercial products like contact lenses [34-40]. Doxorubicin is a chemotherapy agent primarily utilized in the treatment of various cancers, including breast, stomach, ovarian, bladder, lung, etc. [41]. It works by damaging the DNA of cells so they cannot multiply, ultimately leading to the death of cancer cells. Their clinical applications are limited because of its side effects, specifically cardiotoxicity, nausea, hair loss, fatigue, and mouth sores [42-45]. One of the inherent challenges associated with conventional DOX is its lack of specificity, resulting in severe side effects that limit the ability to administer higher concentrations of the therapeutic agent. Utilizing its potent concentration may impede the viability of cancer cells, yet concurrently induces substantial adverse effects on healthy cells and tissues, leading to issues like cardiotoxicity and hypersensitivity.
Poly (HIPEs) can encapsulate the drug molecules and target specific tumor sites more precisely than traditional approaches using free drugs. In addition, they can prevent side effects associated with non-specific distribution of free drugs over time, allowing sustained release and targeted drug delivery when exposed to specific physiological triggers such as pH or temperature. All in all, hydrogels offer a promising platform for the delivery of anticancer drugs due to their stability, controlled release properties, ability for targeted delivery, biocompatibility, and versatility. By harnessing these characteristics, hydrogel-based drug delivery systems hold great potential for improving the efficacy and safety of anticancer therapies.
In this research, hydrogel based on HEMA monomer and Alg via a poly HIPE template (poly (HEMA-co-Alg)) was produced and its optimization steps were estimated by the amount of water absorbed by the resulting polymer. The highest amount of water absorption is indicated by the optimal values of the effective factors (such as concentration of surfactant, monomers, and cross-linker) in the tailoring of holes and windows. The optimal polymer was identified and DOX drug was loaded on it, and then its release was evaluated at different pH and temperatures.
Experimental
Materials
The following reagents were used for polyHIPE production: Cyclohexane (99.5%, Merck), 2-hydroxyethyl methacrylate, (HEMA, 97%, Aldrich), sodium alginate (Alg), N,N′-methylenebisacrylamide (MBA, 99%, Sigma-Aldrich), ammonium persulfate (APS, 98%,Sigma-Aldrich), N,N,N’,N’-tetramethylethylene diamine (TEMED, 99%, Merck), Triton X-100 ( Sigma-Aldrich), ethanol (EtOH, Sigma-Aldrich), sodium chloride (NaCl, Merck), potassium chloride (KCl, Merck), disodium phosphate (Na2HPO4, Sigma-Aldrich), monopotassium phosphate (KH2PO4, Sigma-Aldrich), sodium hydroxide (NaOH, Sigma-Aldrich), hydrochloric acid (HCl, Merck), and distillated water were used as received. Doxorubicin hydrochloride (DOX) was produced by Samen Pharmaceutical Co, Tehran, Iran.
Apparatus
Scanning Electron Microscopy
The porous structure of poly HIPE products was identified by field emission scanning electron microscopy (FE-SEM), model MIRA3 TESCAN Company.
FT-IR
The conformation of porous monolith poly HIPE-based co-polymer hydrogel (poly (HEMA-co-Alg)) was analyzed by Fourier transform infrared (FT-IR) spectrophotometry, which was determined by a Shimadzu IR Prestige-21 spectrometer in the wavelength number region of 400-4000 cm-1 using KBr pellets under hydraulic pressure of 750 kg/cm2.
TGA
The thermogravimetric analysis (LINSEIS STA PT-1000 Germany) was used for the determination of the thermostability of polyHIPE based polymers.
UV-Vis Spectrophotometer
The UV-Vis absorption spectra were determined on Shimadzu UV-visible 2550 PC spectrophotometer.
Methods
PolyHIPE Based Co-Polymeric Hydrogel Synthesis (HEMA-Alg polyHIPE)
The monolithic materials were produced by the radical polymerization of O/W emulsions, characterized by a large volume of internal phase. Initially, Alg solution (3%) was prepared by solving Alg (0.075 g) in deionized water (2.5 mL) mixed for 24 h, and then HEMA (1.172 mL), MBA (0.089, 0.093, 0.117, 0.136, and 0.156 g (0.57, 0.6, 0.75, 0.88, and 1.01 mmol)), APS (0.025, 0.05, 0.1, 0.125 g (0.1,0.2, 0.4, and 0.54 mmol)) and Triton X100 (0.55, 0.73, 0.91, and 1.1 mL) were added to Alg solution. Thereafter, the blend was stirred with a magnetic stirrer (Heidolph, Germany) in a speed of 400 rpm to reach a homogenized solution. Following that, 10.1 mL of cyclohexane (oil or internal phase) was added drop by drop to the mixture at constant stirring speed at 400 rpm to give stable HIPEs (The volume of the oil phase is three times that of the water phase). After completing the addition process, stirring was continued for 20 minutes to obtain a homogeneous thick emulsion.
200 µl of TMED was added into the emulsion with a stirring speed of 600 rpm for 1min and the final stable concentrated emulsion was moved into a small tube and sealed. The tube was put into a 50 ○C water bath and maintained for 4 h to form hydrogel product. The remaining initiator, emulsifier, and stabilizer were washed by Soxhlet extraction in distillated water and then ethanol over two consecutive nights. The poly HIPE was dried in a vacuum oven at 50 ○C in a day.
Bulk Synthesis
The bulk polymer (AL) was synthesized through the same compositions, as (AB polyHIPE) in Table 1, even though here, only water phase ingredients were utilized and polymerized. The resulting bulk polymer was heated in a convection oven over 50 ○C for 24 hours. The monolith was washed with distilled water and ethanol to remove unreacted reagents, and then dried in convection oven at 50 ○C a night.
Water Uptake Studies
The washed and dried polyHIPE samples were broken into pieces of approximately similar sizes and shapes. The samples (30 ± 5 mg), were immersed in 5 mL of distillated water and kept in the closed container at room temperature. Excess water was eliminated with a napkin before reordering the weight of the samples after 1 h, 2 h, and 24 h. In each measurement, triplicate samples were done for a poly HIPE with the same composition. The water uptake percentage (WU) was calculated as follows based on Ws (swelled weight) and Wd (dried weight)
WU (x100) = ((Ws-Wd)/Wd) x100
Drug Loading
Triplicates an optimized polyHIPE (sample AB, in Table 1) was chosen for drug loading and release experiments. Samples were cut into a semi-circular shape with a diameter of approximately 20 mm and weighed (30 ± 5 mg). The dry weight was documented, and the samples were immersed separately in the doxorubicin (DOX) drug with a concentration of 100 mg/mL for 24 h. The amount of added drug solution for each sample was 1 mL per 10 mg of each sample. After 24 h, the samples were withdrawn from the solutions, and excess liquid was removed by filter paper. The loaded polyHIPEs were dried in a vacuum oven at 25 °C overnight. Drug loading was reported as a loaded drug in gram-per-gram polyHIPE.
Table 1: The composition of synthesized poly HIPE
|
Wu (X100) |
Oil phase |
Water phase |
Sample |
|||||
|
Cyclohexane (mL) |
Water (mL) |
Triton X100 (mL) |
APS (g) |
MBA (g) |
HEMA (mL) |
Alg (g) |
||
|
850 ± 10 |
10.1 |
2.5 |
0.91 |
0.0250 |
0.1170 |
1.17 |
0.0750 |
AA |
|
970 ± 15 |
10.1 |
2.5 |
0.91 |
0.0500 |
0.1170 |
1.17 |
0.0750 |
AB |
|
650 ± 08 |
10.1 |
2.5 |
0.91 |
0.1000 |
0.1170 |
1.17 |
0.0750 |
AC |
|
510 ± 05 |
10.1 |
2.5 |
0.91 |
0.1250 |
0.1170 |
1.17 |
0.0750 |
AD |
|
310 ± 05 |
10.1 |
2.5 |
0.91 |
0.0500 |
0.8900 |
1.17 |
0.0750 |
AE |
|
530 ± 05 |
10.1 |
2.5 |
0.91 |
0.0500 |
0.9360 |
1.17 |
0.0750 |
AF |
|
720 ± 08 |
10.1 |
2.5 |
0.91 |
0.0500 |
0.1404 |
1.17 |
0.0750 |
AG |
|
- |
10.1 |
2.5 |
0.91 |
0.0500 |
0.1638 |
1.17 |
0.0750 |
AH |
|
560 ± 06 |
10.1 |
2.5 |
0.73 |
0.0500 |
0.1170 |
1.17 |
0.0750 |
AI |
|
400 ± 05 |
10.1 |
2.5 |
1.1 |
0.0500 |
0.1170 |
1.17 |
0.0750 |
AJ |
|
240 ± 05 |
10.1 |
2.5 |
0.55 |
0.0500 |
0.1170 |
1.17 |
0.0750 |
AK |
|
130 ± 03 |
- |
2.5 |
- |
0.0500 |
0.1170 |
1.17 |
0.0750 |
AL |
|
|
|
|
|
|
|
|
|
|
Drug Release
The release was carried out with the formerly loaded polyHIPEs. Samples were submerged in 5 mL of PBS in pH (7.4 and 5.4) and incubated at 37 °C and 42 °C without agitation. The buffer was replaced with a new solution after 30 min, 1 h, 5 h, 12 h, 24 h, 48 h, and 72 h. The absorption of the collected samples was examined with UV-Vis spectrometry at a wavelength of 480 nm. The amount of drug delivered and its release rate over a period time was estimated by employing a prepared calibration curve. The calibrations curve was calculated using a concentration of 10, 25, 50, 100, 150, and 200 μg/mL of doxorubicin in PBS buffer. The drug release was reported based on the relative percentage of released drugs to the amount of loaded after a specific time.
Results and Discussion
Mechanism of Hydrogel Formation
Copolymerization of HEMA on Alg substrate in an aqueous medium was carried out using a radical reaction, initiated by APS (ammonium persulfate). According to Figure 1, Sulfate anion-radicals are generated by the decomposition of APS persulfate with the aim of TEMED as a co-catalyst. The hydrogen from the hydroxyl of the alginate backbone is abstracted to generate the alkoxy radicals present on the beds. Therefore, this per sulfate saccharide redox system results in active centers on the substrate to radically initiate polymerization of HEMA-co-Alg leading to a graft co-polymer. As a crosslinking agent, e.g., MBA, is present in the system, the co-polymer is comprised of a cross-linked structure Alg.
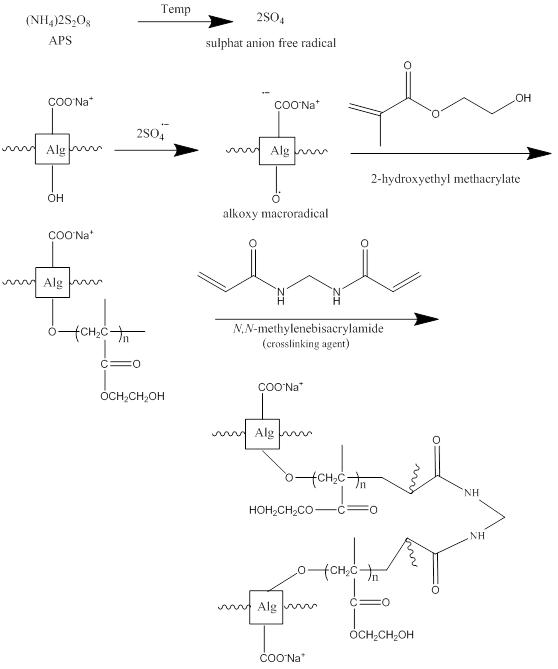
Figure 1: The proposed mechanism for the synthesis of poly HIPE (HEMA-Alg)
Characterization of poly HIPE-based Co-Polymeric Hydrogel
The FT-IR Spectra
The characterization of co-polymer (HEMA-Alg) crosslinked to MBA hydrogel was conducted by FT-IR analyses and the spectra are depicted in Figure 2. According to the FT-IR infrared spectrum, the wavelength range is between 400 and 4000 cm-1. In the spectrum related to Alg, the broad band at 3440 cm-1 is related to the stretching vibrations of the OH group, and two absorption bands at 1610 cm-1 and 1417 cm-1 are related to the COO - group. The absorption band in the region of 2920 cm-1 indicates the stretching vibration of CH on the ring. The absorption band at 1030 cm-1 is related to C-O stretching vibrations, and the absorption band at 819 cm-1 is related to O-Na vibration in sodium alginate [46]. In HEMA spectra, there are 3300 cm-1 and 2960 cm-1 bands which related to OH and CH alkyl stretching frequency, respectively, and the two remaining 1610 cm-1 and 1150 cm-1 was corresponded to C = O and C-O stretching bands, individually [47]. The FT-IR spectrum of the poly (HEMA-Alg) has a new absorption band in the region of 1735 cm-1, which indicates the stretching of the carbonyl ester group. Likewise, the C-O stretching vibrations peak at the region of 1150 was observed with greater intensity in the spectrum of resulting co-polymer which confirmed the creation of hydrogel material.
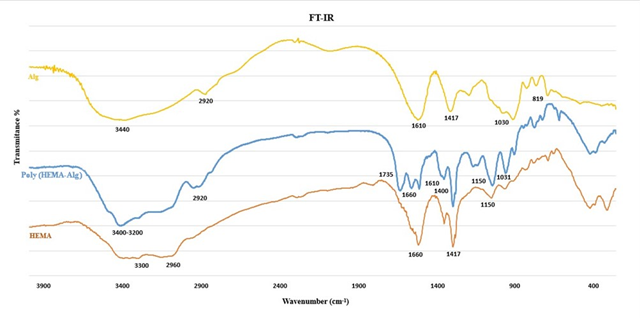
Figure 2: FT-IR spectra of Alg, poly (Alg-HEMA) and HEMA
Surface Morphology
To evaluate the structure and morphology of co-polymer (HEMA-Alg), SEM was used, and the results are demonstrated in Figure 3 (A-I).
Figure 3 (A-F) shows SEM images of optimized poly HIPE (HEMA-co-Alg). The presence of multiple pores and the non-smoothness of the effective surface indicate a high macroporosity in the structure of synthesized polymer in an emulsion environment. Owing to the presence of these porosities, they are able to absorb water and drugs to their surface and interior regions.
Porous observed structures with pores and windows (the visible big pores observed on the surface of porous monolith) were derived from the big bubbles due to vigorous stirring and the Ostwald ripening. Large cavities form as a result of the coalescence of smaller droplets of the dispersed phase with each other, giving rise to larger droplets (Ostwald ripening phenomenon). The images of the block copolymer surface presented in Figure 3 (G-I) have been synthesized without an emulsion environment, with a composition similar to optimized poly HIPE (sample AB in Table 1).
From the comparison of the completely rough, non-porous, and impermeable surface of block co-polymer (sample AL in Table 1) with the porous and interconnected surface of optimized poly HIPE structure, it can be concluded that evacuation of the internal phase (oil phase) caused to form of a highly interconnected, open cell, emulsion-templated and permeable construction. That can be strong evidence to justify the high-water absorption of the final poly (HIPEs).
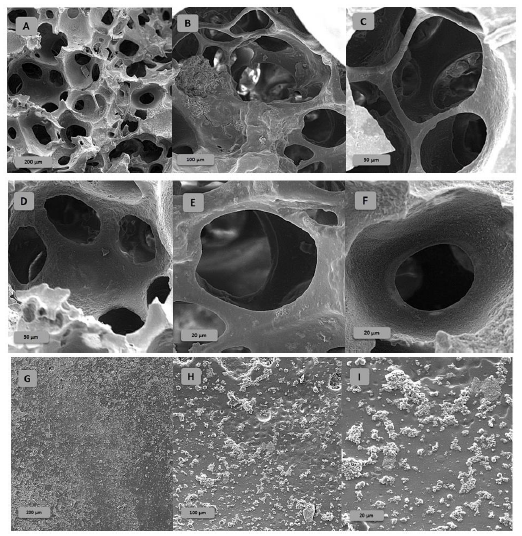
Figure 3: SEM of optimized poly HIPE-based hydrogel with porous structures from lower magnification to higher (a, b, c, and d) compared to smooth bulk polymer hydrogel structure (e, f, and g)
Thermo-gravimetric Analysis
Thermal stability of the resulting poly HIPE was compared with HEMA and Alg in Figure 4 (A) for all samples, a little weight loss was observed around 100 to 150 °C owing to the elimination of their moisture content. The diagram of the HEMA monomer (liquid) showed a steep slope after the reduction of the initial water, which lost all its weight due to the evaporation of the entire molecule at 200 °C. In the Alg graph a weight loss of 15% was experienced until the temperature of 175 °C, and then it suddenly lost 30% of its initial weight again with a steep slope, which can be considered due to the conversion of alginate end groups to CO2- and CO, the evaporation of these molecules. This reduction weight process continued with a gentle slope until the temperature of 550 °C, which led to the loss of another 20% of the mass. This process can be attributed to the breakdown of the natural polymer structure and its internal chains. Finally, 25% of the mass of Alg remained constant and did not decrease until 700 °C. In the thermogram of the manufactured co-polymer, it is evident that the temperature decomposition range increases due to the creation of covalent bonds between monomers, which creates stronger chains. The final polymer lost only 10% of its initial weight up to 300 °C and continued to lose about 80% of its weight again with a steep slope around 450 °C.
This reduction process continued until 550 °C, at which temperature the polymer was completely destroyed. The differential thermogravimetric (DTG) curves, as illustrated in Figure 4 (B) that the maximum decomposition rates occur at 120, 190, and 400 °C for HEMA, Alg, and Poly (HEMA-Alg) respectively. These outcomes obviously signify that the crosslinked network inherent to poly (HEMA-Alg) imparts greater stability compared to individual monomers.
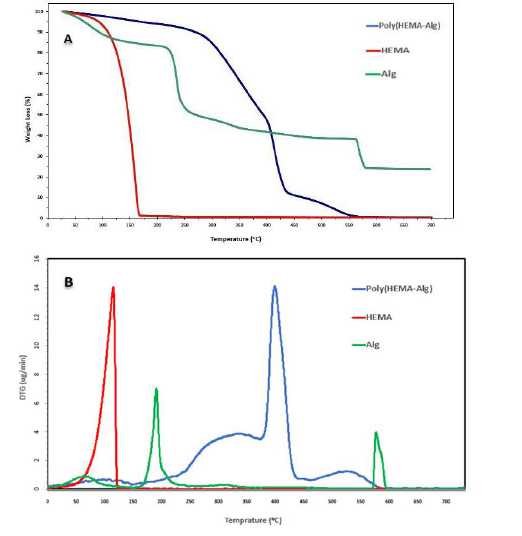
Figure 4: Thermal gravimetric analysis of optimized poly HIPE (AB polymer): (A) TG and (B) DTG
Optimization of Poly HIPE Hydrogel Synthesis
The Effect of Initiator Concentration
In this part of the experiment, the swelling ratios as a function of initiator concentration were investigated (Figure 5). The maximum swelling (970% g/g) has been observed at 0.087 mol/L of APS (optimum value). As the initiator concentration increases, water absorption decreases faster than the optimum value of APS. These results can be due to the creation of a larger number of active free radical sites on the Alg backbones, which led to a higher chance of 2- hydroxyethyl methacrylate graft copolymerization reaction onto the substrates. The decrease in swelling ratios after using of the higher amount of 0.087 mol/L APS may be attributed to an increasing terminating step reaction via bimolecular collision which is referred to as “self-crosslinking” by Chen and Zhao [47]. This fact leads to decreasing molecular weight of grafted hydrogel and swelling value is declining. As a result, by crossing the optimum value, there is an inverse relationship between increasing the amount of initiator and water absorption [48-50].
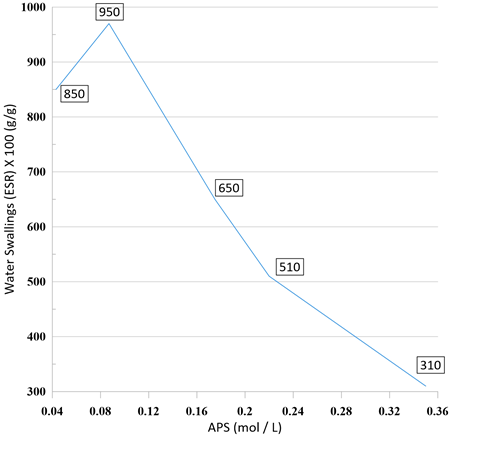
Figure 5: The impact of initiator concentration on water swelling of poly (HIPEs)
The Effect of Cross-Linker Concentration
Changing the crosslinker concentration has a significant effect on the amount of water absorption, due to a direct effect on the shape of the holes and windows. When the MBA concentration was 0.0936 g (8% with respect to HEMA), the water swelling was 530% g/g (Figure 7). As can be seen in Figure 6 (A1-A3), this sample has porous structure consisting of irregularly interconnected spherical voids that have walls with closed windows. This structure is different from the typical poly HIPE structure due to the low MBA content [51-52]. Due to the inadequate quantity of crosslinker, there was insufficient formation of crosslinking bonds among the polymer chains during the polymerization stage. Consequently, a portion of the about 0.2% of oil phase was extruded, leading to the development of a polymer characterized by closed pores.
MBA concentration increased to reach 0.117 g (10% with respect to HEMA) water absorption reached the top of its amount of 9.7 g/g as depicted in Figure 6 (B1-B3) the resulting structure is porous and holes are interconnected like structure of a typical poly HIPE. This Porous structure created from the evaporation of the organic phase, there are holes in diameter, and each hole is interconnected to the other by completely-opened windows [38]. The results obtained from Figures are consistent with the maximum water absorption of this optimum value of cross-linker concentration.
As the concentration of crosslinker increased to 0.1404 g (12% respect to HEMA), the tendency to separate two heterogeneous phases increases, so that the Ostwald ripping [53-55] is strengthened by increasing the percentage of crosslinker. In this phenomenon, small droplets combine to create larger droplets, as can be seen in Figure 6 (C1-C3). Upon evaporation of the oil phase, the transformation of large droplets into macroscopic voids occurs, accentuating the Ostwald ripening phenomenon.
This progression correlates with an augmented count of macroscopic voids, consequently diminishing the specific surface area of the resultant polymer. Increasing the percentage of crosslinkers to 14% created very heterogeneous and fragile polymers which caused dissociated parts when immersed in water. Therefore, there is a very narrow compositional window for crosslinker concentration that can be used to prepare a homogeneous and highly porous poly HIPE.
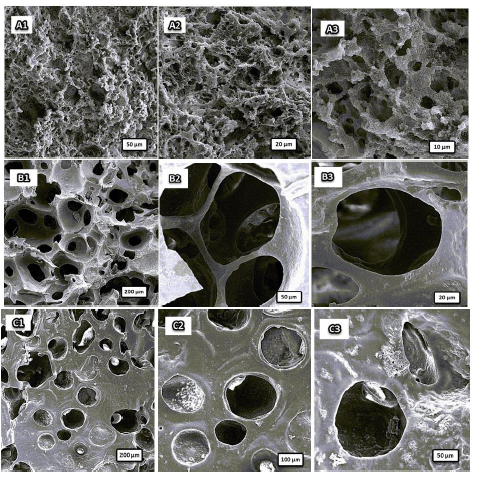
Figure 6: SEM micrographs of different concentration of MBA (A1-A3) 0.0936g, (B1-B3) 0.117 g, and (C1-C3) 0.1404 g
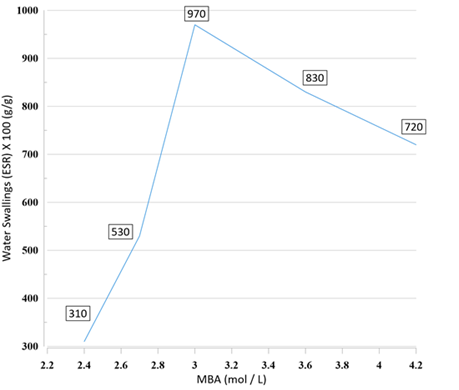
Figure 7: The impact of cross-linker concentration on water swelling of poly (HIPEs)
The Effect of Surfactant Concentration
When the surfactant volume was equal to 0.73 mL (20% with respect to the aqua phase), water absorption of 560% g/g was obtained (Figure 8). At this stage, due to the low concentration of surfactant, a slightly unstable emulsion was created sample AI in Table 1. By increasing its amount to 25% (0.91 mL (sample AB in Table 1)), water absorption reached a maximum of 970% g/g.
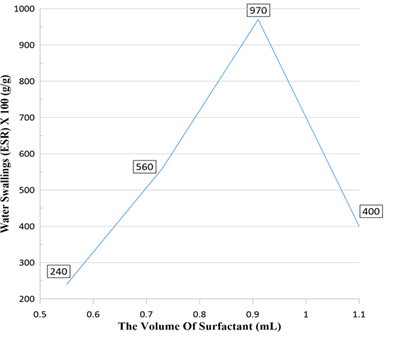
Figure 8: The impact of surfactant volume on water swelling of poly (HIPEs)
Normally, increasing the concentration of surfactant reduces the droplet size and leads to the formation of a larger number of interconnected holes [56]. By increasing the surfactant concentration again to 30% (1.1 mL (sample AJ in Table 1)), the viscosity of the solution increased. Therefore, the surface tension forces produced during the homogenization process, which will not be able to break the droplets into smaller sizes. It will create larger pores with closed windows, which can explain the decrease in water absorption.
Drug Release Study
To investigate the effect of the pH-responsive manner of synthesized hydrogel on controlled drug delivery, the well-known anticancer drug, DOX was selected for in vitro experiments. A test was carried out in PBS of pH 7.4 (normal cells) and 5.4 (tumor cells) at two different temperatures. As it can be seen in Figure 9, a rapid release behavior was observed for all samples. Most of the release happened in the first 24 hours, which was expected due to the microporous structure of synthesized materials. The highest amount of drug (nearly 88.95%) was released at pH 5.4 and 42 °C. However, at the temperature and pH of normal body cells (pH 7.4 and 37 °C), the least release has occurred (38.63%), as the temperature goes up; the drug becomes more soluble at a pH of 7.4. Nevertheless, even with increased solubility, it still cannot be released as effectively as it would at a pH of 5.4, especially at lower temperatures, which can be said that the poly HIPE substrate loaded with drugs works specifically for cancer cells. When drug molecules are confined within a hydrogel structure, they can be liberated using various methods. Initially, following Fick's law, the process involves the infiltration of solvent molecules into the hydrogel package, resulting in swelling. Subsequently, the specific way in which the molecules are released could be influenced by how readily the drug dissolves at different pH of solutions.
The favorable factor of DOX solubility in an acidic environment can be seen as a strong point in this research because of the protonation of DOX amino molecules in an acidic environment, it can be attributed to their rapid solubility here. At higher pH, because of the hydrogen bonds between hydroxyl groups of alginate and HEMA, and DOX amino groups, they have entered into an attraction of the drug with the hydrogel network, and the drug molecules are trapped in the bed, so less release occurs [57].
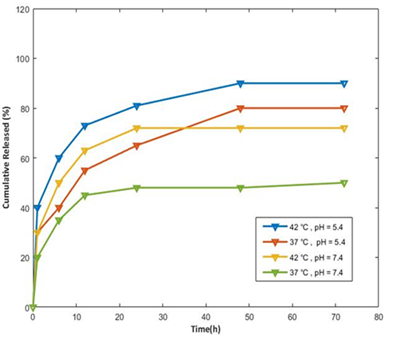
Figure 9: Drug release of poly (HEMA-Alg) at pHs 5.4 and 7.4 at 42 and 37 °C
Conclusion
In this study, 2-hydroxyethylmethacrylate was grafted onto the sodium alginate chain through free radical polymerization in emulsion template to produce unique pH-responsive poly (HEMA-Alg) hydrogel for selective release of doxorubicin as cancer therapeutics. Reducing the pH from 7.4 to 5.4 notably hastened the release rate of DOX. Even after 48 hours, only 38.63% and 63.09% of DOX were released from drug-loaded beds at pH 7.4 and 78.08, respectively, whereas a substantially higher release of 88.95% was achieved within the same timeframe at pH 5.4. It is worth noting that biodegradable moieties such as Alg and HEMA have been utilized here to enhance biocompatibility with the physiological conditions. In addition, during the synthesis of the hydrogel, variations in concentrations of surfactant, cross-linker, and initiator were examined to obtain an optimum sample for drug release studies. Moreover, synthesized optimized hydrogel with suitable functional groups can facilitate the slow release of DOX which demonstrates the maximum release in higher temperature and lower pH (cell tumours condition). To sum up, it can be said that the combination of biocompatibility, versatility, controlled release, targeted delivery, and drug protection makes hydrogels an important feature in drug delivery systems, offering significant advantages for the effective and safe delivery of therapeutic agents in various biomedical applications.
Conflict of interest
The authors declare that they have no conflict of interest in this study.
Ethical Approval
No experiments are conducted on animals or humans.
Availability of Data and Material
Not applicable.
ORCID
Hassan Hassani
https://orcid.org/0000-0002-0210-5194
Ghasem Rzanejad Bardaiee
https://orcid.org/0000-0001-7331-0059
HOW TO CITE THIS ARTICLE
Samira Kianpour, Hassan Hassani, Ghasem Rezaejnade Bardajee. Synthesis of Porous Polymer with Biocompatible Sodium Alginate and 2-Hydroxyethyl Methacrylate Monomers in High Internal Emulsion as Drug Delivery Substrate in Releasing of Doxorubicin. Chem. Methodol., 2024, 8(3) 200-216
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company