Document Type : Original Article
Authors
1 Department of Chemistry, Graduate University of Advanced Technology, Kerman, Iran
2 Environment Department, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran
Abstract
We applied GCE modified with Ce3+-NiO hexagonal nanoparticles (Ce3+-NiO HNPs) for preparing methyldopa electrochemical sensor. Electrochemical study of the modified electrode showed that within the optimal conditions of phosphate buffer solution (PBS) at a pH of 7.0 in CV, oxidation potency of methyldopa decline to ~100 mV at the modified electrode in comparison with an unmodified GCE. The sensor exhibits a sensitive response to methyldopa in range between 0.1 and 80.0 μM with a limit of detection (LOD) equal to 0.03 μM. In addition, as sensing materials for simultaneously determine hydro-chlorothiazide and methyldopa, Ce3+-NiO HNPs/GCE, exhibited high sensitivity. The defined and separated oxidation peaks of a mixture of methyldopa and hydrochlorothiazide were obtained with significant peak potential differences of 390 V. The proposed electrochemical sensor was employed in analysis of both drugs in the real specimens.
Graphical Abstract
Keywords
- Glassy carbon electrode
- Ce3+-NiO hexagonal nanoparticles
- Methyldopa
- Hydrochlorothiazide
- Electrochemical sensor
Main Subjects
Introduction
High blood pressure (HBP) or hypertension has been considered as one of the diseases that with its insidious development results in numerous risks for humans' health and develops wider therapeutic arsenal for minimizing the patients' morbidity. In this regard, methyldopa has been considered as a major medicine to treat it [1]. The drug may enhance the heart rate and narrow the blood vessels [2]. For this reason, methyldopa has been proposed as one of the competitive inhibitors of dopadecarboxylase that metabolizes L-dopa into DA [3, 4]. Put differently, increased dosage of methyldopa increases risks of headache, weakness as well as drowsiness. Thus, determination of methyldopa in pharmaceutical as well as biological samples is very important. Hydrochlorothiazide has been introduced as one of the anti-hypertensive drugs, helping the excretion of surplus water and salt from the human body. Moreover, researchers have considered it as one of the benzothiazide diuretic medications with the direct function on the kidneys, which obstructs renal sodium chloride channel and enhances excretion of sodium chloride and partly of potassium ions [5]. This drug has a widespread utilization in treating edema and cardio-vascular disease, managing diabetes insipidus, and controlling HBP [6, 7]. Additionally, half-life of hydrochlorothiazide changes from 6-15 h and about 50% to 60% of the orally taken medicine that is excreted through urine. Above 90% of the absorbed medicine is excreted as the unmodified medicine via urine. An over-dose of hydrochlorothiazide of the patients' loss fluid and electrolyte and the reported symptoms have been dizziness, sedation or impaired consciousness, hypo-tension as well as cramps in the muscles [8]. Hence, it would be important to devise novel techniques for rapid and accurate detection of hydrochlorothiazide. The above compounds are important drugs in treatment of hypotensive. So, authors focused on the development of precise and reliable analytical methods for simultaneous detection of the drugs in biological and pharmaceutical specimens. Analytical methods have attracted more attention in recent years in separation and detection systems [9-15].
Numerous analytical techniques have been presented to detect methyldopa and hydrochlorothiazide, that are HPLC [16, 17], spectrophotometry [18, 19], fluorescence [20], gas chromatography [21], chemiluminescence [22], capillary electrophoresis [23], liquid chromatography/tandem mass spectrometry [24], chemometry [25], and electrochemistry [26-30]. Amongst them, electrochemical methods are highly prioritized due to their merits like operation simplicity, lower expense of instruments, fast response, possible miniaturization, and appropriateness for the real-time determination [31-33]. Modifying the electrode surfaces may be accompanied by several benefits in electrochemical responses as well as electrochemical research [34-37]. Several specific features may be viewed for modifying the electrodes with the use of nanomaterials, such as catalysis, larger specific surface areas and greater adsorption locations. Therefore, applying nanomaterials in constructing the electrochemical sensors experienced the recent increase [38-42].
As a result of specific features of metal oxide NPs, researchers have been highly attracted by using Co3O4, NiO, ZnO, CuO, and etc for modifying the electrodes' surface [43-47]. Amongst the above NPs, researchers have investigated NiO, which is one of the p-type semiconductors with the wider bandgap equal to 3.7 eV under room temperature and higher isoelectric point (IEP) of nearly 10.7, [48, 49] due to its higher chemical stability, electrocatalysis, electron transfer capabilities as well as acceptable biological compatibility [50-52].
However, for electrochemical applications, the elevation in conductivity has been recognized as one of the basic parameters for improving its electro-chemical functions. For improving electrical features of metal oxides, we commonly performed heteroatom doping. In general, metallic dopants would enter lattice of metal oxides for enhancing its electrocatalytic behaviors [53]. As an instance, numerous studies have confirmed doping the heteroatoms like Li+, La3+, Co3+ and Y3+ for improving its electrocatalytic behaviour for diverse uses [54-56]. Herein, we used rare earth ions (Ce3+) doping for enhancing electrocatalytic function of NiO nanostructures.
The electrocatalytic activity of Ce3+/NiO HNPs towards methyldopa would be addressed. Outputs suggested higher electrocatalytic activities of Ce3+/NiO HNPs toward this compound. Also, the proposed electrochemical sensor was used to simultaneously determine methyldopa and hydrochlorothiazide. Moreover, analytical function of the modified electrode for quantifying methyldopa and hydrochlorothiazide in the real samples was assessed.
Material and methods
In this step, electrochemical measurements were done using an Auto-lab potentiostat/galvanostat (PGSTAT 302N, Eco Chemie, the Netherlands). Measurements were performed at room temperature, with a single component 3-electrode cell that had a platinum auxiliary electrode and an Ag/AgCl (3 M KCl) reference electrode. Ce3+/NiO HNPs/GCE was used as the working electrode and a Metrohm 827 pH-meter was used for controlling the pH of solutions. Each chemical was of analytical reagent grade that had been bought from Merck Company in Darmstadt, Germany. In addition, we applied doubly distilled-water. Methyldopa, hydrochlorothiazide and all other reagents were of analytical grade and obtained from Merck chemical company.
Preparation of the electrode
For preparing the Ce3+-NiO HNPs modified GCE, 1mg of Ce3+-NiO HNPs was dispersed in 1 mL distilled water and ultrasonicated for nearly thirty minutes. Then, 4 μL of the suspension was coated on the GCE surface and finally dried at the room temperature.
Real sample preparation
Five methyldopa tablets (labeled 250 mg) were ground. Then, the tablet solution was prepared by dissolving 250 mg of the powder in 100 mL water by ultrasonication. Then, different volume of the diluted solution was transferred into a 10 mL volumetric flask and diluted to the mark with phosphate buffer (pH 7.0).
Five hydrochlorothiazide tablets (labeled 50 mg) were ground. Then, the tablet solution was prepared by dissolving 50 mg of the powder in 100 mL water by ultrasonication. Then, different volume of the diluted solution was transferred into a 10 mL volumetric flask and diluted to the mark with phosphate buffer (pH 7.0).
When the urine samples were collected, we used a refrigerator to directly store them and centrifuged 10 ml of the samples at 2000 rpm for fifteen minutes. We used a 0.45 µm filter for filtering out the supernatant and transported various amounts of the solution into a 25 mL volumetric flask for diluting to the mark with PBS at a pH of 7.0. Afterwards, we used various contents of methyldopa and hydrochlorothiazide for spiking the diluted urine samples. Methyldopa and hydrochlorothiazide amounts were analyzed using our new sensor with the standard addition procedure.
Result and Dissection
Electrochemical behaviour of methyldopa at the surface of various electrodes
The effects of the pH of solution on the oxidation responses of Ce3+-NiO HNPs/GCE for 150.0 μM methyldopa was carefully investigated by CV in 0.1 M PBS in various pH-values (2.0 < pH <9.0). It is notable that under pH 7.0 of PBS, we observed the best voltammetric responses; i.e., the largest peak current. Finally, we chose pH 7.0 in 0.1 M PBS for other analytical experimentations. The oxidation mechanism of methyldopa is presented in Scheme 1.

Scheme 1: Mechanism of methyldopa oxidation
The cyclic voltammograms (CVs) recorded by bare GCE, as shown in Figure 1, curve a, and Ce3+-NiO HNPs as shown in curve b, were carried out between using 50 mVs-1 scan rate in 0.1M PBS at a pH of 7.0, including 150.0 μM methyldopa. The anodic peak potential for methyldopa oxidation at the bare GCE was ~500 mV in comparison to 400 mV for on the Ce3+-NiO HNPs/GCE. Moreover, the current value had increased in modified electrode which revealed that the Ce3+-NiO HNPs/GCE had electrocatalytic behavior to the methyldopa oxidation.

Figure 1: Cyclic voltammograms of (a) bare GCE and (b) Ce3+/NiO HNPs/GCE in 0.1 M PBS (pH 7.0) in the presence of 150.0 μM methyldopa the scan rate 50 mVs -1
Effect of potential scan rate
We determined the effects of the potent scan rate on the oxidation current of methyldopa (Figure 2). Enhancing the potential scan rate elevated the peak current. Additionally, the oxidation process was diffusion-controlled due to the linear dependence of the anodic peak current (Ip) on the square root of the potent scan rate (ν1/2) within a wider ranges between 10 and 1000 mVs−1 (Figure 2, inset).
Chronoamperometric analysis
With the confirmation of diffusion process during the methyldopa oxidation process on the surface of Ce3+-NiO HNPs/GCE, the chronoamperometric method with applied potential of 0.45 V was employed for determining diffusion coefficient (D) of methyldopa (Figure 3). Experimental outputs of I versus t −1/2 were drawn (Figure 4 A), with the best fits for various concentrations of methyldopa. In the next step, we plotted final slopes that corresponded to the straight lines in Figure 4A, plotted against methyldopa concentration (Figure 4B). Finally, mean value of D was computed 2.5×10−6 cm2/s based on the Cottrell equation and final slope [64].

Figure 2: Linear sweep voltammograms of Ce3+/NiO HNPs/GCE in 0.1 M PBS (pH 7.0) containing 250.0 µM of methyldopa various scan rates; numbers 1–13 correspond to 10, 30, 70,100, 200, 300, 400, 500, 600,700, 800, 900 and 1000 mV s-1. Inset: Variation of anodic peak current vs. square root of scan rate
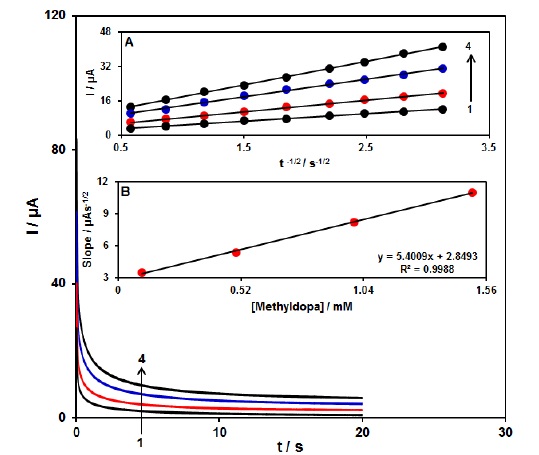
Figure 3: Chronoamperograms obtained at Ce3+-NiO HNPs/GCE in 0.1 M PBS (pH 7.0) for different concentration of methyldopa. The numbers 1–4 correspond to 0.1, 0.5, 1.0 and 1.5 mM of methyldopa. Insets: (A) Plots of I vs. t -1/2 obtained from chronoamperograms 1–4. (B) Plot of the slope of the straight lines against methyldopa concentration
The calibration curve and limit of detection
In this step, we used DPV for investigating voltammetric sensor of Ce3+-NiO HNPs/GCE towards detecting methyldopa detection (Step potential=0.01 V and pulse amplitude=0.025 V). Figure 4 shows the differential pulse voltammograms of methyldopa with diverse concentrations in 0.1 mol L-1 PBS at a pH of 7.0. In Figure 4 (inset), we showed linear enhancement of the oxidation current with methyldopa concentrations in range from 0.1–800.0 μM. Moreover, linear regression equation has been expressed as Ipa=0.0243C(μM)+0.5581 (R2=0.9995) and LOD of methyldopa equaled 0.03 μM.

Figure 4: Differential pulse voltammograms of Ce3+-NiO HNPs/GCE in 0.1M PBS (pH 7.0) containing different concentrations of methyldopa. Numbers 1–13 correspond to 0.1, 5.0, 10.0, 30.0, 70.0, 100.0, 200.0, 300.0, 400.0, 500.0, 600, 700 and 800.0 µM of methyldopa. Insets: (A) a plots of the electrocatalytic peak current as a function of methyldopa concentration in the range of 0.1-800.0 µM
The features of the proposed Ce3+-NiO HNPs/GCE was compared with the values of some in previous works for determination of methyldopa by different methods [27, 58-62]. Table 1 shows the comparison in terms of type of sensor, used technique, linear dynamic range (DLR) and limit of detection (LOD).

Simultaneous determination of methyldopa and hydrochlorothiazide
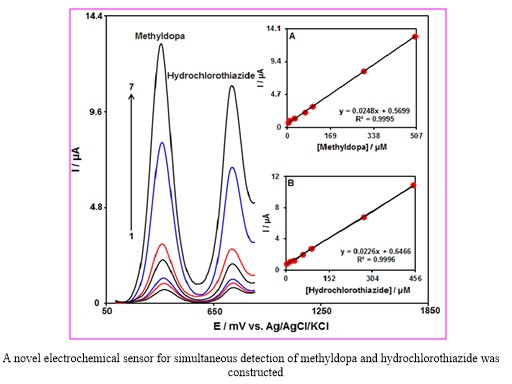
The present research mainly aimed at the simultaneous detection of both methyldopa and hydrochlorothiazide. Therefore, we simultaneously changed the concentration of methyldopa and hydrochlorothiazide, and recorded DPV (Step potential=0.01 V and pulse amplitude=0.025 V). According to the voltammetric outputs, we observed the completely organized anodic peaks at the potential equal to 360 and 750 mV, which corresponded to methyldopa and hydrochlorothiazide oxidation, suggesting the feasibility of simultaneous detection of the compounds (Figure 5). Moreover, sensitivity of the modified electrode for oxidizing methyldopa equaled 0.0248 µAµM-1 that was highly close to the value observed in the absence of hydrochlorothiazide (0.0243 µA.µM-1, Figure 4), revealing independence of the oxidation processes of the compounds at the Ce3+-NiO HNPs/GCE as well as feasible simultaneous detection of the mixtures without any significant interference.

Figure 5: Differential pulse voltammograms of Ce3+-NiO HNPs/GCE of in 0.1 M PBS (pH 7.0) containing different concentrations of methyldopa and hydrochlorothiazide, from inner to outer: 5.0+2.5, 10.0+ 15.0, 30.0+30.0, 700.0+60.0, 100.0+90.0, 300.0+275.0 and 500.0+450.0 respectively. Insets: (A) plot of Ip vs. methyldopa concentrations and (B) plot of Ip vs. hydrochlorothiazide, concentrations
Real sample analysis
For evaluating practical application of Ce3+-NiO HNPs/GCE, we used it to determine methyldopa and hydrochlorothiazide in real samples (methyldopa tablet, hydrochlorothiazide tablet, and urine). DPV response of the samples was identified by some experiments and then methyldopa and hydrochlorothiazide concentrations were added. In the next stage, we used standard addition for quantitative analysis of solutions so that recovery ranged from 97.3-103.0% (Table 2). Outputs reflected the possible feasibility of our new electrode in the real samples with acceptable confidence level.

Conclusion
Electrochemical detection of methyldopa on a GCE modified with Ce3+-NiO HNPs was reported. Moreover, oxidation peak currents of methyldopa remarkably elevated following the electrode modification, which may be caused by catalytic activities and conductivity of Ce3+-NiO HNPs. This electrochemical sensor displayed acceptable sensitivity and broader linear concentration ranges for determination of methyldopa between 0.1 and 800.0 μΜ, with the sensitivity equal to 0.0243 μA μM-1. LOD was also calculated to be 0.03 μM. Also, the results indicated that Ce3+-NiO HNPs/GCE facilitate the concurrent detection of methyldopa and hydrochlorothiazide with reasonable selectivity and sensitivity. Hence, this electrode may electro-chemically differentiate sensing of methyldopa and hydrochlorothiazide. For practical application, Ce3+-NiO HNPs/GCE was employed for sensing of methyldopa and hydrochlorothiazide in the real specimens.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
HOW TO CITE THIS ARTICLE
Sajedeh Salari, Hadi Beitollahi. Sensitive Simultaneous Detection of Methyldopa and Hydrochlorothiazide on Ce3+-NiO Hexagonal Nanoparticles-Modified Electrode, Chem. Methodol., 2021, 5(5) 407-415