CiteScore2024
Web of Science
Document Type : Original Article
Authors
University of Baghdad, Department of Chemistry, Baghdad, Iraq
Graphical Abstract
Keywords
Subjects
Introduction
Triazoles are five membered heterocyclic compounds with the chemical formula of C2H3N3. Triazole has two isomers: 1,2,3-triazole and 1,2,4-triazole. The tautomeric forms of 1,2,4-triazoles are 1H and 4H-1,2,4-triazole is a pharmacologically significant nucleus [1,2]. Antibacterial, antifungal, anticancer, anti-inflammatory, antioxidant, antiviral, analgesic, anticonvulsant, anti-HIV, and biological properties were all found in 1,2,4-triazole derivatives [3-5]. Imidazole is a five-member heterocyclic ring with three carbon atoms and two nitrogen atoms in the positions number one and three. The behavior of one nitrogen is similar to that of a pyrrole- type nitrogen, while the other is similar to that of a pyridine-type nitrogen [6-7]. In drug development, the imidazole nucleus is an essential synthesis approach. The strong therapeutic effects of imidazole-related drugs have encouraged scientists to develop plenty of new chemotherapeutic medications [8,9]. According to the literature review, there is no evidence on the synthesis and investigation of the antibacterial and anticancer activities of these newly synthesized compounds [I-VI]. We synthesized and characterized novel compounds containing N-aceyl, N-thiourea and imidazole units in the same symmetrical 1,2,4-Triazole molecule and also studied their antibacterial and anticancer activities in the hopes of developing new therapeutics.
Material and Methods
General
The chemicals were provided from Aldrich, Merck and GCC Chemicals Co. On (Ir prestige-21) a Shimadzo, Fourier Transform Infrared Spectrometer (FT-IR) spectra were recorded using KBr discs; Bruker, Ultra Shield (500MHz), Switzerland, was used to record 1HNMR spectra.
General Synthetic Procedures
Synthesis of 3,5-bis (4-methoxyphenyl)-4H-1,2,4-triazole-4-amine [I]
4-methoxybenzohydrazide (6.64 g, 0.04 mol) was dissolved in dimethyl sulfoxide (10 mL), refluxed for 17 hrs., then distilled under reduced pressure, cooled, and put into ice water [10,11]. The mixture was stirred for 12 hours at room temperature, filtered and washed with water, dried and recrystallized from aqueous ethanol (1:1).
Synthesis of (E)-4-(1-((3,5-bis(4-methoxyphenyl)-4H-1,2,4-triazol-4-yl) imino)ethyl)aniline [II]
For eight hours, a mixture of the new compound [I] (2.69 g, 0.01 mol) and 4-aminoacetophenone (1.35 g, 0.01 mol) was refluxed in absolute ethanol (10 mL) with some drops of glacial acetic acid [12]. The reaction mixture was cooled to room temperature, then the solid was filtered and purified from ethanol.
Synthesis of 4-((E)-1-((4-((E)-1-((3,5-bis(4-methoxyphenyl)-4H-1,2,4-triazol-4-yl)imino) ethyl)phenyl)imino)ethyl)phenol [III]
Equimolar amounts of 4-hydroxyacetophenone (1.36 g, 0.01 mol), compound [II] (4.13 g, 0.01 mol) were refluxed for 8 hrs. in 10 mL absolute ethanol with three drops of glacial acetic acid [13]. The resulting precipitate was filtered, dried, and the residue purified with ethanol.
Synthesis of N-(3,5-bis (4- methoxyphenyl)- 4H-1,2,4-triazol-4- yl)-N-(1-chloro -1-(4-(N-(1-chloro-1-(4-hydroxyphenyl) ethyl) acetamido) phenyl) ethyl) acetamide [IV]
To a stirred solution of compound [III] (5.31 g, 0.01 mol), 10 mL dry benzene was added drop by drop of acetyl chloride (0.025 mol) in an ice water bath with constant stirring for an hour [14]. After that, the reaction was refluxed for two hours. The solvent was evaporated, and the white precipitate was gently washed with water before being purified from diethyl ether.
Synthesis of (R)-1-(N-(4-((R)-1-(N-(3,5-bis(4-methoxyphenyl)-4H-1,2,4-triazol-4-yl)acetamido)-1-(carbamimidoylthio)ethyl)phenyl)acetamido)-1-(4-hydroxyphenyl)ethyl carbamimidothioate [V]
To synthesize thiourea derivatives [V] [14], a mixture of compound [IV] (6.88 g, 0.01 mol), thiourea (1.52 g, 0.02 mol), anhydrous sodium carbonate (1.06g,0.01mol) and 20 mL acetone was heated for 6 hrs. with stirring. After cooling, the reaction solution was put into ice water, filtered and recrystallized from ethyl acetate.
Synthesis of N-(3,5-bis (4-methoxyphenyl) -4H- 1,2,4-triazol-4-yl)-N-((R)-1-((4,5-diphenyl-1H-imidazol-2-yl)thio)-1-(4-(N-((R)-1-((4,5-diphenyl-1H-imidazol-2-yl)thio)-1-(4-hydroxyphenyl)ethyl) acetamido) phenyl) ethyl) acetamide [VI]
2-Hydroxy-1,2-diphenylethan-1-one (5.32 g, 0.02 mol) was added to a stirred solution of compound[V] (7.67 g, 0.01 mol) in dry DMF (10 mL). For 7 hrs., the reaction mixture was refluxed [15]. After cooling, a few drops of cold water were added and stirred until a precipitate. The crystals were filtered, dried and purified from ethyl acetate.
Biological Evaluation
Antibacterial Activity:
The antibacterial activity of the synthesized compounds [I-VI] was tested by using the Well Diffusion Method [16], against three types of bacteria: Staph. aureus (G+), Klebsiella pneumoniae (G-)and Pseudomonas aeruginosa (G-) and compared with Ampicillin, as antibiotic standard. All the tested compounds were dissolved in DMSO. The plates were then incubated at 37 °C and examined after 24 hrs.
Cytotoxicity Activity
The compounds [II] and [VI] were screened for their cytotoxicity activity, using three types of cancer cell liens: MCF-7 (human breast carcinoma cells), Hep G2 (human liver cancer cell line) and WRL-68(the human hepatic cell line). Freshney's protocol for cell culture media, reagents, and solutions were followed [17]. The viability of MCF-7, HepG-2 and normal cell line WRL-68 cells, after adding various concentrations of compounds [II] and [VI] was determined by using an ELISA reader at a wavelength of 575 nm, respectively.
Result and Dissection
Scheme 1 shows the synthetic routes for the synthesis of derivatives [I-VI].

Scheme 1: Synthesis of compounds [I-VI]
The acid hydrazide (4-methoxybenzohydrazide) was condensed in the presence of dimethyl sulfoxide to produce the novel 3,5-bis (4-methoxyphenyl)-4H-1,2,4-triazole-4-amine [I]. Refluxing equimolar of 4-amino triazole [I] with 4-amino acetophenone in ethanol with some drops of glacial acetic acid yielded the new Schiff base [II], at the same procedure Schiff base [III] was synthesized. The new N-acetyl, N-thiourea and diphenyl imidazole derivatives [IV-IV] were synthesized by three-step reactions. N-acyl derivative [IV] was synthesized by addition reaction of acetyl chloride with Schiff base [III] in dry benzene. The following is an outline of the reaction mechanism suggested [14], as shown in Scheme 2:

Scheme 2: Proposed mechanism for the synthesis of N-acyl derivative [IV]
The second step involved the reaction of thiourea with N-acyl [IV] in anhydrous sodium carbonate in analar acetone to yield N- thiourea compound [V]. The next mechanism is proposed for this reaction [14], Scheme 3:
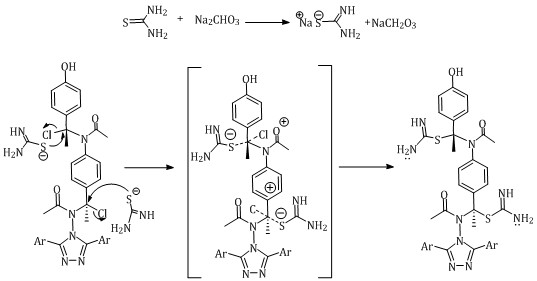
Scheme 3: Proposed mechanism for the synthesis of N- thiourea derivative [V]
The cyclization reaction of N-thiourea derivative with 2-hydroxy-1,2-diphenylethan-1-one in DMF caused the formation of a new imidazole derivative [VI] by intermolecular nucleophilic substitution. Scheme 4 illustrated the suggested mechanism for obtaining the desired product [14].

Scheme 4: Proposed mechanism for the synthesis of imidazole derivative [VI]
Physical properties and spectral data were used to indicate the structures of all synthesized compounds, as shown in Table 1. These findings provide strong support for the formation of the structure for these molecules.
Biological Evaluation
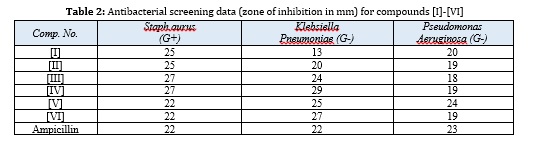
The synthesized compounds [I-VI] showed good to moderate antibacterial activity against three types of bacteria. Compounds [IV], [V] and [VI] showed excellent inhibition of these bacteria, as shown in Table 2.
The cytotoxicity activity of compound [II] showed significant effects at concentration 400 μl /ml against MCF-7 cell line Table 3, whereas the viability cells were (37.81%,24.19%,12.27, 5.71%,4.05% and 5.13%) in concentration (400, 200, 100, 50, 25, 12.5µg/ml), respectively, and IC50=206.1, as shown in Figure 1.
Also, the cytotoxicity activity of compound [VI] showed significant effects at concentration 400 μl /ml against Hep G2 cell line Table 4, whereas the viability cells were (61.23%, 54.47%,38.39 % , 20.87%, 4.28% and 5.98 %) in concentration (400, 200, 100, 50 , 25, 12.5µg/ml), respectively, and IC50=82.60, as shown in Figure 2.

The results of this study showed the compounds [II] and [VI] affecting against MCF-7 and Hep G2 cancer cells (in vitro), respectively, and at high concentration did not affect the growth of normal cells WRL-68, which confirms the safety of the utilize of compounds in drugs used according to a dose-dependent treatment. This efficacy needs to be studied, detected and analyzed in future studies on factors that inhibit the growth of cancer cells [18,19].


Figure 1: Cytotoxic activity of compound [II] on MCF-7 cell line

Figure 2: Cytotoxic activity of compound [VI] on Hep G2cell line
Conclusion
The aim of the research was to create N-aceyl, N-thiourea, and imidazole derivatives by a series of reactions that began with symmetrical 4-amino-1,2,4-Triazole. The antibacterial activity of all of the synthesized compounds [I-VI] was tested in vitro. MCF-7, Hep G2 and WRL-68; cancer cell lines were used to estimate the cytotoxic effect of different concentrations of the created compounds[II] and [VI].
Acknowledgments
The authors extend sincere thanks and appreciation to Prof. Dr. Issam M.A. Shakir and Prof. Dr. Nagam S. Turkey, for their appreciable advice in the research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Conflict of Interest
We have no conflicts of interest to disclose.
HOW TO CITE THIS ARTICLE
Shamsalmiluk Mohammed Abdulghani, Muna Sameer Al-rawi, Jumbad Hermiz Tomma. Synthesis of New 1,2,4-Triazole Derivatives with Expected Biological Activities, Chem. Methodol., 2022, 6(1) 59-66
DOI: 10.22034/chemm.2022.1.6
| Article View | 6,550 |
| PDF Download | 1,785 |