Document Type : Original Article
Authors
Chemistry Department, Payame Noor University, 19395-4697, Tehran, Iran
Abstract
This study utilized potassium permanganate and natural polymers starch and gelatin as stabilizing agents to the green fabrication of manganese dioxide nanoparticles MnO2-NPs for photocatalytic degradation of methylene blue. MnO2-NPs were characterized using UV-Vis spectroscopy, FT-IR, and X-ray diffraction spectroscopy, as well as Field Emission Scanning Electron Microscopy (FESEM). XRD confirmed the amorphous nature and purity of the nanoparticles. The photocatalytic activity of MnO2-NPs was examined by the degradation of methylene blue dye under neutral pH. Results showed 95% dye degradation within 45 minutes under repeat cycling, indicating the excellent photocatalytic performance. The green synthesis method and effective photocatalytic activity demonstrate of starch and gelatin-stabilized MnO2-NPs as sustainable photocatalysts for degradation of organic pollutants. The produced potential in the two beds with starch and gelatin was examined.
Graphical Abstract
Keywords
Main Subjects
Introduction
In recent years, there has been an increase in the growth of textiles, leather, paint, food, plastics, and cosmetics, which are linked to the disposal of different amounts of organic pollutants that are hazardous to the environment, aquatic systems, and human health [1]. Owing to their high catalytic activity, nontoxicity, chemical stability, affordability, optical and electrical qualities, and environmentally favorable attributes, TiO2 and ZnO are among the most frequently used photocatalysts [2, 3]. Nanocatalysts can help achieve this objective of green chemistry, which is concerned with healthy chemical reactions that produce products that are secure and cause the least amount of pollution (consumption of chemical resources and energy) [4]. The integration of nanotechnology with green chemistry will become increasingly popular in coming decades. Nanotechnology is a revolutionary science that remains in its infancy. Although there are many traditional methods for synthesizing nanoparticles, green synthesis methods are more efficient than physical and chemical approaches [5]. Several metal nanoparticle syntheses utilizing yeast have been described using green nanotechnology [6], fungi [7], bacteria[8], algae [9], and plant extracts [10]. Designing, developing, and expanding procedures that reduce or remove potentially harmful substances for people or the environment is the best description of "green chemistry" [11, 12]. There are many types of factors that pollute natural waters, including population growth, industrialization, etc. The main sources of wastewater pollution in these industries are various dyes and organic pollutants. These dyes are a serious threat to human health [13]. Recently, due to the effect of chemical and physical reactions that occur between organic (polymer) and inorganic components (MnO2-NPs), MnO2-NPs are also widely used for dye removal. The selection of a suitable photocatalyst should be based on low cost, easy synthesis, band gap, high ability to work under sunlight or other visible light, efficiency, easy recovery, and reuse [14]. Photocatalytic breakdown of dyes using MnO2 nanocatalysts and UV light [15]. Photocatalysts are a group of catalysts that can be activated by specific wavelengths of UV light to convert pollutants in water or air into safe substances such as water and carbon dioxide and these photocatalysts show low efficiency under the sun light because UV constitutes only 3% of the total solar energy [16]. Manganese oxides, in particular, have drawn much attention owing to their unique physical and chemical characteristics and use in biosensors, ion exchange, molecular adsorption, and secondary batteries for energy storage [17]. In this study, manganese dioxide nanoparticles (MnO2 –NP) were employed as photocatalyst for the degradation of methylene blue (MB) under UV light [2]. The goal of this research is to synthesize nanoparticles in a green manner using polymer materials that are safe, eco-friendly, and easy to prepare. MnO2-NPs are highly effective in the degradation of MB in the presence of UV light and are used to eliminate pollutants [18]. In this study, it has been tried to use polymer materials and plant extracts for the nanoparticles synthesis, which is a new and safe synthetic method, which is recently widely used for green synthesis.
Materials and Methods
Potassium permanganate, methylene blue, and other precursors for the MnO2-NPs production were bought from Merck and Aldrich companies and were utilized directly. Commercially available ingredients used in this experiment were gelatin and starch. Double-distilled water was used as a solvent. The ambient pH was controlled using hydrochloric acid and commercial NaOH. In photocatalytic tests, the organic dyes are the intended contaminants. A stock solution of MB was prepared by dissolving the required quantity of the dye in deionized water. Different concentrations were created from the stock solutions to investigate photocatalytic degradation. All UV-Vis absorption spectra were captured using a Japanese-made spectrophotometer (Shimadzu Model 2550). Powder samples smaller than 50 nm were used to obtain X-ray diffraction spectra. In this experiment, a TESCAN MIRA3-FEG scanning electron microscope was used to obtain images of the nanoparticles. The fluid and nanoparticles were separated using a HETTICH centrifuge (Model 420 EB). To examine the photocatalytic capabilities of the nanoparticles, an Osram model UV-A lamp with 11-watt radiation intensity was employed.
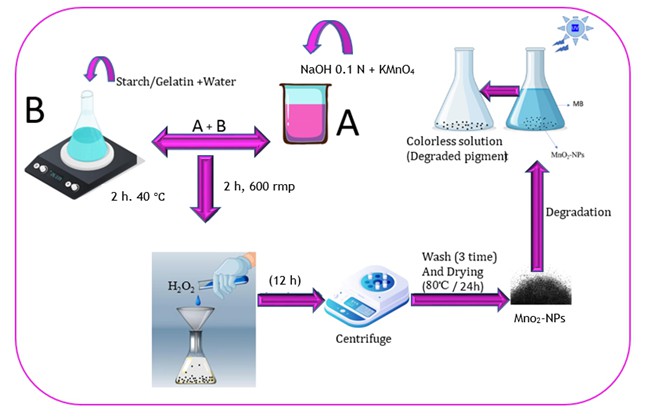
Fabrication of MnO2-Nps
For the MnO2-NPs synthesis, 0.79 g of potassium permanganate as a precursor and 50 ml of 0.1 N NaOH solution were placed in a container and mixed with a magnetic stirrer for 30 minutes. Gelatin or starch (0.5 g) in 50 ml of distilled water was prepared at 40 °C for 2 h to obtain a clear solution. To obtain a homogenous mixture, the polymeric solution was added to the purple permanganate mixture and stirred for 2 h at 600 rpm, and then 20 ml of 15% H2O2 solution was added dropwise to turn the solution colorless and deposit a black precipitate. Next, the mixture was centrifuged after forcefully mixing it for 12 hours. The precipitate washed three times with distilled water and dried at 80 °C for 24 hours before being stored for identification.
Photocatalytic Study
The photocatalytic performance of the nanoparticles was assessed by measuring the change in absorbance at 400-700 nm caused by the photocatalytic degradation under UV light. MnO2-NPs (10-3 M) were added to 50 ml of MB (10-5 M) solution. The photocatalytic activity of the MnO2-NPs was studied, and the removal of aqueous MB from aqueous media demonstrated that these nanoparticles had a particular influence on color degradation. Nanoparticles with smaller sizes and higher activity levels are more effective for pigment degradation [19]. The solution was kept in the dark, and the quantity of adsorbed MB was determined using a UV-Vis spectrophotometer. After irradiation for 5 min, the absorbance spectra were recorded every 15 min [20].
The degradation of MB by MnO2-NPS at pH 7 under UV light is shown in Figures 1a (gelatin bed) and 1b (starch bed). To investigate the photocatalytic process, the contact time was used as a variable, with a time interval of 15 min from 0 to 90 min.
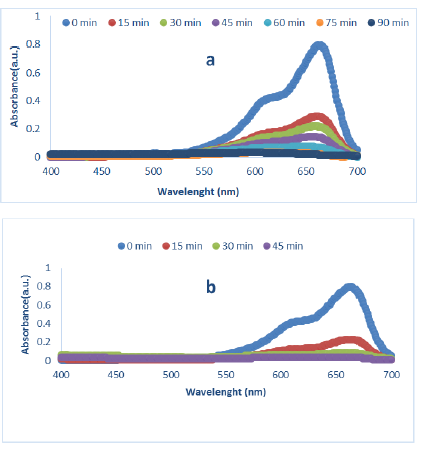
Figure 1: Degradation spectra of MB (10-5M) under UV light in the presence of MnO2-NPs, synthesized at a: gelatin bed and b: starch bed (pH:7, MnO2-NPs (10-3 M))
The Influence of pH on Photocatalytic MB Degradation
The pH parameter is an effective parameter in the photocatalytic process, and we explored the optimization of the pH parameter for nanoparticles generated in gelatin and starch beds. The pH ranges of 5, 7, and 9 were used for this purpose, with starting concentrations of MB (10-5M) and MnO2-NPs (10-3M), as indicated in Table 1. The suspension was exposed to UV-A (11 W) light for 0-90 minutes, with a 15-minute time interval measured using a spectrophotometer. As presented in Table 1, the amount of photocatalyst degradation increased at pH 7, and the percentage of MB degradation at this pH was estimated to be greater than 90% [21]. Table 1 shows the percentage of MB breakdown by nanoparticles generated on gelatin and starch beds at pH values of 5, 7, and 9 [13]. A neutral pH creates more efficiency and better conditions for pigment degradation. It was observed that pigment degradation occurred in a much shorter time (approximately 95% decolorization in 45 min) [22]. Figure 2 displays a comparison between the percentage of MB degradation and color degradation time for the nanoparticles synthesized on starch (a) and gelatin (b) substrates. The duration was approximately 90 min, and the amount of pigment degradation was approximately 90% for the nanoparticles synthesized in the gelatin substrate and approximately 45 min, and the amount of pigment degradation was approximately 95% for nanoparticles synthesized in the starch bed.
Table 2: MB degradation in gelatin and starch bed at pH 5, 7 and 9
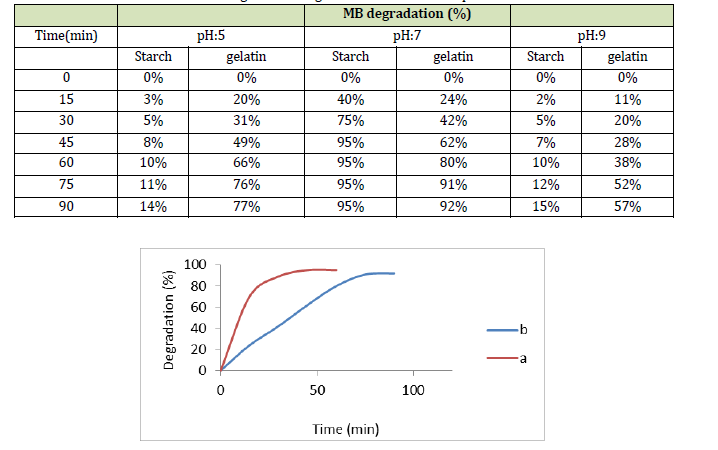
Figure 2: Degradation percentage of MB by synthesized nanoparticles in starch bed (a) and gelatin bed (b)
Result and Discussion
This study explored using manganese dioxide nanoparticle (MnO2-NPs) as a photocatalyst to degrade methylene blue dye in wastewater under UV irradiation. Experiments optimized the conditions for dye photodegradation finding pH 7, MnO2-NPs catalyst concentration of 10-3 M, and a methylene blue concentration of 10-5 M at room temperature. An array of characterization methods such as FT-IR, UV-Vis, FESEM, EDX, and XRD were employed to fully profile the green-synthesized manganese oxide nanoparticles. The green synthesis method produced smaller, more efficient nanoparticles than traditional chemical methods [23]. For example, plant-derived TiO2 nanoparticles degrade methylene blue better than chemically produced TiO2 nanoparticles. The plant extracts provide hydroxyl groups (OH·) that facilitate photocatalytic reactions when exposed to UV light, generating hydroxyl and superoxide (O2·-) radicals that break down dyes [24].
Characterization of MnO2-NPs
XRD Evaluation
XRD Spectrum of MnO2-NPs was obtained at room temperature. Figure 3 shows the XRD pattern of the prepared MnO2-NPs with broad peaks at 11.8o, 38.3o, and 64.8o which matches well with the δ-MnO2 JCPDS value (JCPDS No. 80-1098)[25].
All the above reflections can be indexed to the corresponding crystal planes ((hkl) (001), (111), and (005)) [26]. The broad peaks indicate that the sample was a weakly crystalline. The crystallite size was designed in agreement with the Debye–Scherrer formula, as described in Equation (1).
![]()
Where, D is the particle size (nm), k = 0.94 is the crystallized form factor, is the wavelength (0.154 nm), is the highest width at half maximum of the peak (rad), and is the diffraction angle (o). The size of nanoparticles in starch and gelatin substrate was observed to be about 31.36 and 44.51 nm, respectively.
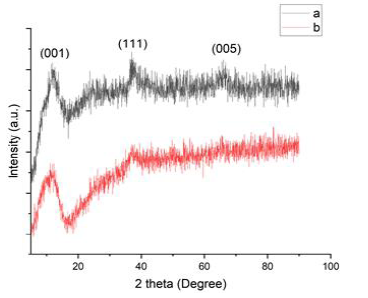
Figure 3: XRD Spectrum of MnO2-NPs at starch bed (a) and gelatin bed (b)
FT-IR Analysis
The peaks of the gelatin at 3330 cm-1 were attributed to the presence of hydrogen bond water and amide-A, 1680 cm-1 peaks corresponding to the occurrence of amide-I and peaks ranging from 1400 cm-1 to 1380 cm-1 were attributed to the symmetric and asymmetric bending vibrations of the methyl group[27]. Starch peaks at approximately 1000 cm-1 to 1200 cm-1 are characteristic of C-O-H bonds. The bonds in the region between 3360 cm-1 and 2880 cm-1 characterize the stretching of the OH and CH bonds, respectively. At 1680 cm-1, there is a flexion bond of flexion of the OH of water, indicating the polymer is hygroscopic. At 1480 and 1200 cm-1 the C-H bending vibrations and the transmittance band between 1000 and 920 cm-1 are characteristic of polysaccharides and are attributed to the strain deformations of the C-O-C flexion of the OH [28]. The peaks observed between 600 cm−1 and 900 cm−1 represented C-O, C-C, and C-O-C stretching, and C-O-H and C-H bending modes of the polymer backbone. For the MnO2-NPs, the vibration peaks corresponding to the spinel structure were identified at approximately 560, 660, and 1120 cm-1. The broad band at 3387 cm-1 was believed to be associated with the stretching vibrations of hydrogen-bonded surface water molecules and hydroxyl groups. The band located at 560 cm-1 can be ascribed to the MnO vibrations of the MnO2 nanopowder. The spectrum did not show the organic groups found in the MnO2-NPs [23].
FESEM/EDX Studies
The surface morphologies of the MnO2-NPs synthesized from the polymeric substrates were investigated using FESEM and EDX. FESEM images of the MnO2-NPs on the polymer substrates are presented in Figures 4 (a: gelatin bed) and (b: starch bed), respectively. The images showed that the MnO2-NPs grew uniformly and had a spherical shape. The size of the nanoparticles synthesized in gelatin and starch substrates is approximately 67.18 nm and that in starch substrate is approximately 47.1 nm [4]. Mn and oxygen were detected in the EDX spectra [29].
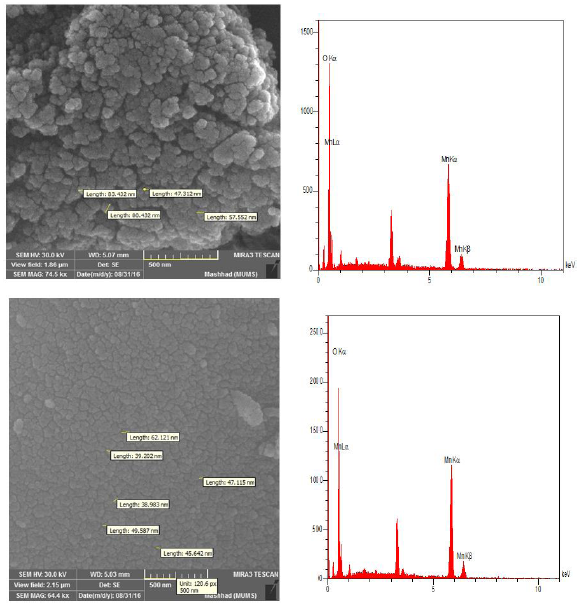
Figure 4: SEM and EDX image of MnO2-NPs in gelatin bed (a) and starch bed (b)
Conclusion
In this study, MnO2-NPs nanoparticles were synthesized using the green polymers gelatin and starch to control nanoparticle size and apply green chemistry principles. Analysis confirmed successful MnO2-NPs synthesis. Experiments found a 10-3 M photocatalyst concentration and 10-5 M methylene blue at neutral pH and room temperature optimal for MB degradation. Nanoparticles synthesized in gelatin degraded approximately 92% of MB in 90 minutes. Nanoparticles synthesized on the starch degraded approximately 95% of MB in 45 minutes. Reusing the nanoparticles reduced degradation efficiency by about 20% over three cycles. Starch-synthesized nanoparticles were 31.36 nm in size vs. 44.51 nm for gelatin-synthesized particle by XRD. However, FESEM imaging measured larger nanoparticle of 67.18 nm (gelatin) and 47.1 nm (starch). Overall, starch-synthesized nanoparticles performed the fastest MB degradation under neutral conditions, demonstrating a promising green photocatalyst for wastewater treatment.
Acknowledgments
This study is supported by Payame Noor University of Tehran, Iran based on the Ph.D dissertation.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed toward data analysis, drafting, and revising the paper and agreed to responsible for all the aspects of this work.
Conflict of interest
The authors declare that they have no conflicts of interest in this article.
ORCID
Mohammad Hakimi
https://orcid.org/0000-0001-8179-1622
Behrouz Elhaminezhad
https://orcid.org/0009-0004-3158-6136
HOW TO CITE THIS ARTICLE
Mohammad Hakimi, Hasan Ali Hosseini, Behrouz Elhaminezhad. Fabrication of Manganese Dioxide Nanoparticles in Starch and Gelatin Beds: Investigation of Photocatalytic Activity. Chem. Methodol., 2024, 8(1) 37-46
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER