Document Type : Original Article
Authors
1 Department of Chemistry, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran
2 Polymer Research Laboratory, Department of Polymer Science and Engineering, University of Bonab, P.O. Box 5551395133, Bonab, Iran
Abstract
A simple approach for component reaction between aldehydes, anilines or ammonium acetate, and dimedone in ethanol as a solvent is studied using Ag-SiO2 nanoparticles. This approach results for the synthesis of various decahydroacridines in appropriate yields (80-94%). The use of Ag-SiO2 nanoparticles as a heterogeneous catalyst lets a clean procedure.
Graphical Abstract
Keywords
Main Subjects
Introduction
Multi-component reactions (MCRs) are the effective pathway in present chemistry because of their substantial features for example conventional reaction planning and atom economy. MCRs ease the synthesis of target products of pharmacological and biological significance by presenting numerous steps in one-pot reaction [1-7].
In cyclic di-carbonyl compounds the situation of C=O groups concludes completely the effortlessness of their cyclization and produces them as suitable found at ion for the preparation of sulfur-, oxygen-, and nitrogen-including heterocycles [8,9].
One of the most investigated types of heterocyclic compounds is decahydroacridine diones. The existence of numerous reaction moieties in decahydroacridinediones increases wide synthetic potentials [10]. Decahydroacridinediones include a 1,4-dihydropyridine ring as basic part and are accessible by several types of Hantzsch synthesis [11–14], which is a basic fragment of progressively significant NADH, NADPH, and N–alkylnicotinamides [15]. Decahydroacridinediones display a wide range of pharmacological and biological [16,17] activity, containing antimicrobial properties [18,19] such as antimicrobial [20,21], anticancer [22], cytotoxic [23], antitumor [24], anti-multidrug-resistant [25], fungicidal [26], and broadly approved as calcium blockers [27,28]. Several approaches have been considered for the produce of these target products by using numerous catalysts like Cu(II) [29], [MIMPS]3PW12O40 and [TEAPS]3PW12O40 Schiff base [30], Ag nanoparticle [31], Glutathione-Coated Magnetic Nanoparticles [32], Fe3O4 nanoparticles [33], and cobalt-alanine metal complex [34].
The nanoparticles are highly applicable in various industries such as electronics, mechanics, and chemistry. They further have potentially been applied in different technologies that deal with catalysts, engineering materials, superconductors, and magnetic materials [35-41]. The nanoparticles of metal oxides have attracted an extraordinary attention. In multiple chemistry fields, they can provide conventional materials with feasible but unusual modifications. In different organic syntheses, these nanoparticles have been mainly employed as nano-catalysts, thanks to their suitable surface area versus their corresponding compounds in bulk form [42-47].
Silica-coated particles are a category of materials broadly studied in numerous fields of materials and colloid science [48-52]. Mesoporous silica, owing to its proper hydrothermal stability, large surface functionality, and area has been revealed widely for biomedical uses, specifically intracellular drug delivery [53–55]. Ag nanoparticles engrossed significant consideration owing to their conducting, catalytic, and optical properties [56,57]. Silica-supported silver nanoparticles (Ag@SiO2 core–shell) were studied as an appropriate catalyst in the field of chemistry [58–60].
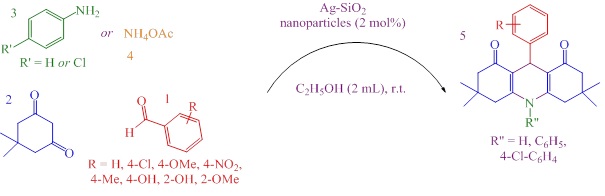
In viewpoint of the above-mentioned reports and our studies for the produce of heterocyclic products and application of metal oxide nanoparticles catalysts [61-66], herein, we report novel catalytic applications of Ag-SiO2 nanoparticles for the preparation of decahydroacridine 5 via the one-pot three-component reaction of aldehydes 1 with dimedone 2 and anilines 3 or ammonium acetate 4 in ethanol solvent at room temperature (Scheme 1).
Experimental
Reagent and analysis
The reagents were prepared from Merck and Aldrich. Melting points were measured on a Thermo Scientific apparatus and are uncorrected. FT-IR spectra were attained by a FT-IR Bruker (WQF-510) spectrometer. 1H-NMR spectra were achieved by Bruker DRX‐400 MHz spectrometer. The spectra were measured in CDCl3 relative to TMS (0.00 ppm). Chemical shifts are measured in ppm. Reactions were observed by TLC on aluminum sheets silica gel F254. The products are characterized by linking their melting points, FT-IR, and 1H-NMR and with those studied in published papers.
Scheme 1: synthesis of decahydroacridineby usingcore–shell Ag-SiO2 nanoparticles
General procedure for the preparation of Ag-SiO2 nanoparticles
Silica particles were prepared using sol-gel method. Therefore, a solution of ethanol (100 mL), hydrochloric acid 1% (5 mL) and water (1.98 g; 110 mmol) was prepared. The resulting solution was stirred for 5 min, and then TEOS (10.41 g; 50 mmol) was added dropwise and stirred for 2 h. The resulting SiO2 nanoparticles were stabilized. The silver nitrate was dissolved in minimal water. Next, the prepared silver solution was added to the silica nanoparticles solution and stirred for 10 h to prepare a colorless catalyst solution [67].
General procedure for the produce of decahydroacridinesby using Ag-SiO2 nanoparticles
A mixture of aldehydes (1 mmol), anilines (1 mmol), or ammonium acetate (0.077 g; 1 mmol), dimedone (0.280 g; 2 mmol), and Ag-SiO2 nanoparticles (2 mol%) at room temperature in ethanol as a solvent (2 mL) was stirred for proper time (Table 2). TLC was used to monitor the reaction progress (ethyl acetate: n-hexane; 2:5). After end of the reaction, the products were isolated by filtration and extracted with CH2Cl2. Thereafter, the organic layer was separated, the solvent was vaporized, and the basic solid purified by recrystallization from ethanol.
Selected spectral data analysis for products
9,10-Bis(4-chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (Table 2, entry 5):White solid; M.P.: 287-290 oC, Yield: 90%; FT-IR (KBr) (νmax, cm-1): 3043, 2955, 1663, 1341, 1220; 1H-NMR (400 MHz, CDCl3): δ = 0.96 (s, 6H), 1.08 (s, 6H), 1.76–2.18 (m, 8H), 5.20 (s, 1H), 7.13-7.53 (m, 8H).
10-(4-Chlorophenyl)-3,3,6,6-tetramethyl-9-(4-nitrophenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (Table 2, entry 6): White solid; M.P.: 258–260 oC; Yield: 94%; FT-IR (KBr) (νmax, cm–1): 2954, 1675, 1510, 1340, 850; 1H-NMR (400 MHz, CDCl3): δ = 1.14 (s, 6H), 1.26 (s, 6H), 2.38-2.50 (m, 8H), 5.57 (s, 1H), 7.27 (d ,4H, J= 6.6), 8.16 (d, 4H, J= 6.7).
Results and Discussion
Synthesis of decahydroacridine by using Ag-SiO2 nanoparticles as a catalyst
In this study, an expedient and benign process was investigated for the preparation of decahydroacridine using Ag-SiO2 nanoparticles as catalyst.
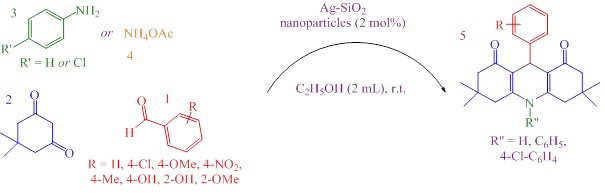
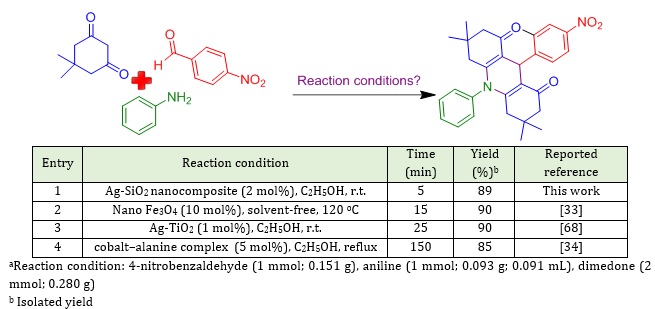
To assess the effectiveness of Ag-SiO2 nanoparticles, the condensation reaction of 4-nitrobenzaldehyde with dimedone and aniline for the produce of corresponding decahydroacridine was studied as a typical reaction.
The model reaction was studied by using several molar ratios of Ag-SiO2 nanoparticles in ethanol at room temperature (Table 1, entries 1-4). Applying 2 mol% of catalyst resulted in higher yield, while the reaction time became shorter (Table 1). In these conditions, the chosen product was achieved in 89% yield within 5 minutes (Table 1, entry 3). Likewise, when the chosen reaction was approved by 3 mol% of the nano catalyst the yield decreased to 83% (Table 1, entry 4). Optimization was lastly attained at 2 mol% when not much difference in the yield was detected after increasing the amount of Ag-SiO2 nanoparticles up to 3 mol%. However, reducing the catalyst level from 2 mol% to 0.5 mol% (as presented in Table 1, entry 1) led to a reduction in desired product yield, and an increase in reaction time. Correspondingly, when the reaction was performed without the catalyst, the intermediates remain unreacted was formed. SiO2 was further used to evaluate the optimized reaction, where in a reduction in yield (47%), and an increase in reaction time (60 minutes) was observed.
We investigated the effect of several solvents on the selected reaction by using 2 mol% of Ag-SiO2 nanoparticles at room temperature. This reaction was achieved in several solvents for example water, chloroform, acetonitrile, and ethyl acetate (Table 1, entries 5-8). The best results were obtained regarding reaction time and yield attained in ethanol (Table 1). It was observing that polarity of solvent plays a significant role for the reaction achievement. In organic solvents and water, the yield of target product was lower and longer reaction time were necessary, while the reaction by using ethanol led to in suitable yield. Based on the results, among all solvents, the best results obtained from application of ethanol, regarding the target product yield and reaction time.
In further effort, after approving the reaction in reflux condition, no changes observed in the terms of desired product yield and reaction time. Therefore, it was establishing that there was not a correlation between reaction rate and temperature. The quantities of reactants, required to synthesize the desired product, were investigated. Applying 1:2:1 molar ratio of aldehydes, dimedone, and anilines, or ammonium acetate, respectively, obtained the maximal yield.
Table 1: Optimization studies for the preparation of desired decahydroacridine
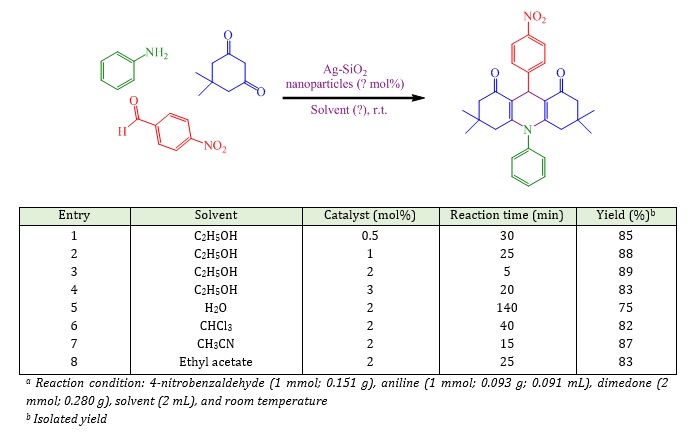
Later on, the reaction of several aldehydes with dimedone and anilines or ammonium acetate verified the overview and scope of the present process. In all cases, the appropriate yields were achieved as revealed in Table 2. The nature and electronic properties of derivatives on the ring were found effective in respect with yield and time of reaction. As exposed in Table 2, electron-withdrawing substituents in aldehydes enhanced the reaction rate while electron-releasing substituents shrank the reaction rate. In addition, the amines reactivity displays the order: 4-chloroaniline > aniline > ammonium acetate.
Table 2: Synthesis of 1,8-dioxo-decahydroacridines using Ag-SiO2 nanoparticles
a Reaction condition: aldehyde (1 mmol), anilines (1 mmol), or ammonium acetate (1 mmol; 0.077 g), dimedone (2 mmol; 0.280 g),Ag@SiO2 core–shell nanoparticles (2 mol%), C2H5OH as a solvent (2 mL), and room temperature
b Isolated yield
Scheme 2 depicts the potential mechanism by which the synthesis of 1,8-dioxodecahydroacridines has been occurred using Ag-SiO2 nanoparticlesis [68]. Initially, the Ag-SiO2 nanoparticles activate the carbonyl group of aromatic aldehyde 1. Indeed, one molecule of dimedone 2 entered in a reaction with an aldehyde 1, which resulted in generating an intermediate 6 by removing one molecule of water. Next, another molecule of dimedone 2 stepped in a reaction with 6 via Michael addition to generate intermediate 7. The intermediate 8 was achieved by nucleophilic attack of anilines or ammonia (in situ produced by ammonium acetate) to activated carbonyl group of 7 by removal of second molecule of water which tautomerized to intermediate 9. Finally, the nucleophilic attack of –NH group on C=O group cyclized the intermediate 9 and generate1,8-dioxodecahydroacridines 5 by eliminating the third molecule of water.
The advantage of current method over reported methods was investigated by comparing the attained results with those reported previously (Table 3). The reaction conditions for the synthesis of target molecule (Table 2, entry 3), were compared considering mol% of the catalyst, reaction time, temperature, and yields.
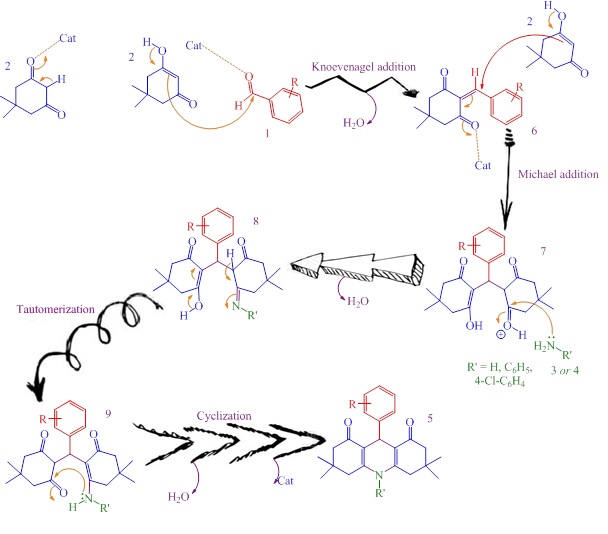
Scheme 2: Suggested mechanism in the preparation of decahydroacridines using Ag-SiO2 nanoparticles
Table 3: Comparison of this approach with further processes for the preparation of target molecule (Table 2, Entry 3)a
Conclusion
In conclusion, a gentle and convenient procedure was developed to generate decahydroacridines from numerous aldehydes, anilines or ammonium acetate, and dimedone using Ag-SiO2 nanoparticles at room temperature in ethanol as a solvent. This procedure includes simple filtration, mild reaction condition, operational ease, good yield of products, and use of Ag-SiO2 as an effective catalyst.
Supporting Information
The supporting data include products' spectral images of FT-IR and 1H-NMR of products.
Acknowledgements
The authors are grateful for the facilities provided to conduct the present study in the chemistry research laboratory at Ayatollah Amoli Branch, Islamic Azad University.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all the aspects of this work.
ORCID
Mehdi Hatami
https://orcid.org/0000-0003-3970-2651
HOW TO CITE THIS ARTICLE
Morteza Farajpour, Seyed Mohammad Vahdat, Seyed Meysam Baghbanian, Mehdi Hatami. Ag-SiO2 Nanoparticles: Benign, Expedient, and Facile Nano Catalyst in Synthesis of Decahydroacridines. Chem. Methodol., 2023, 7(7) 540-551